4 1 The Importance of ATP Adenosine TriPhosphate




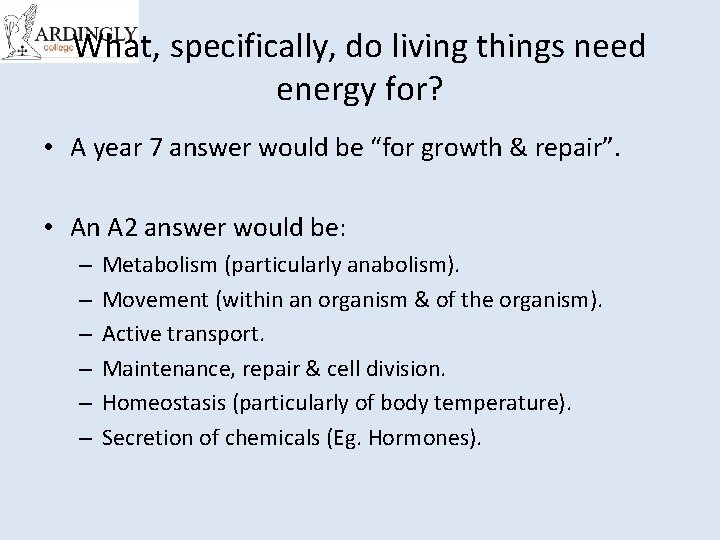







- Slides: 43

4. 1 The Importance of ATP Adenosine Tri-Phosphate

You should be able to discuss… • (a) The importance of chemical energy in biological processes. The central role of ATP as an energy carrier and its use in the liberation of energy for cellular activity. Structure of ATP • (b) The synthesis of ATP by means of a flow of protons through the enzyme ATP synthetase. Chemiosmosis and electrochemical gradient. The similarity between mitochondrial and chloroplast membrane function in providing a proton gradient for ATP synthesis. • (c) The maintenance of the proton gradient by proton pumps driven by electron energy. The alternate arrangement of pumps and electron carriers to form the electron transport chain. (Names of proton pumps and electron carriers in the electron transport system are not required).

Energy “energy can be neither created nor destroyed. However, energy can change forms, and energy can flow from one place to another” • In what forms can you find energy?

Energy’s many forms • There are many different forms of energy. • Can you name some of them: Light Magnetic potential Heat Sound Atomic Gravitational potential Electrical Chemical potential Kinetic Elastic potential

• Green Plants can convert light into chemical energy • All living organisms can convert energy in one form into another • Energy is measured in Joules
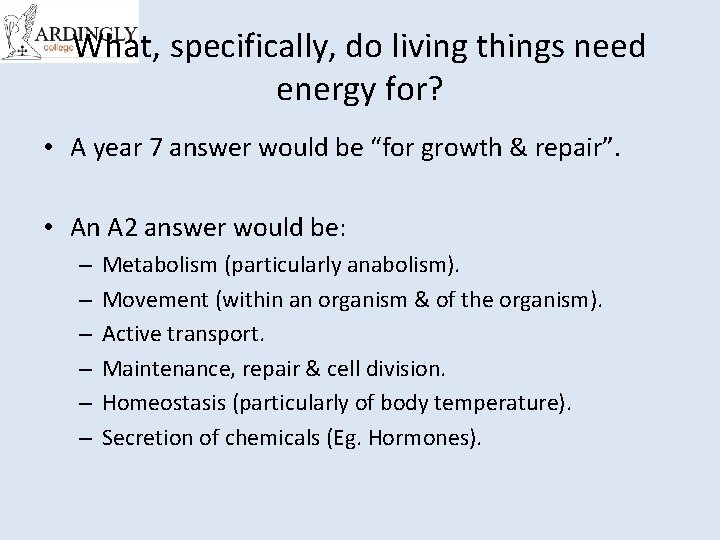
What, specifically, do living things need energy for? • A year 7 answer would be “for growth & repair”. • An A 2 answer would be: – – – Metabolism (particularly anabolism). Movement (within an organism & of the organism). Active transport. Maintenance, repair & cell division. Homeostasis (particularly of body temperature). Secretion of chemicals (Eg. Hormones).

• If you had to “buy” energy, how much would you pay? • Around £ 0. 79 - £ 0. 81 • Its all about ATP!!! (hee hee)

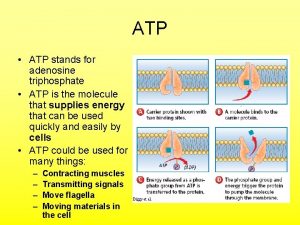
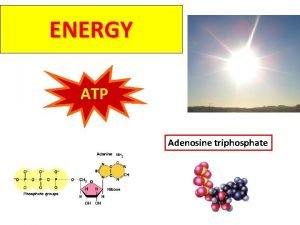
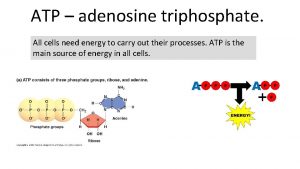
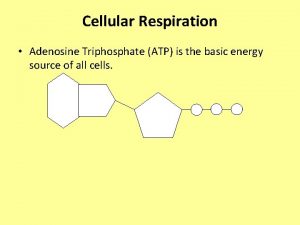
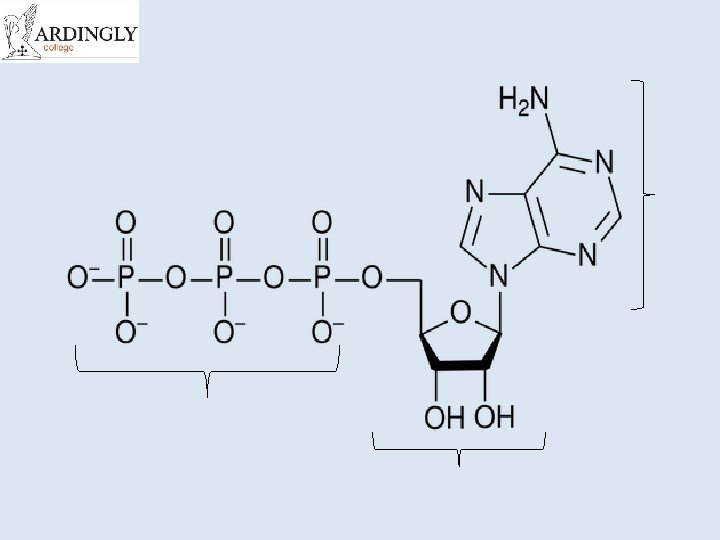
ATP • Adenosine Triphosphate (ATP) is the main energy currency of living cells. • ATP is a small, water soluble molecule. – It is therefore easily transported around the cell. • ATP stores energy as chemical potential energy. – Think of it as a tiny loaded spring. • “Universal Energy Currency” in all living things

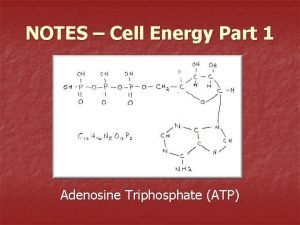
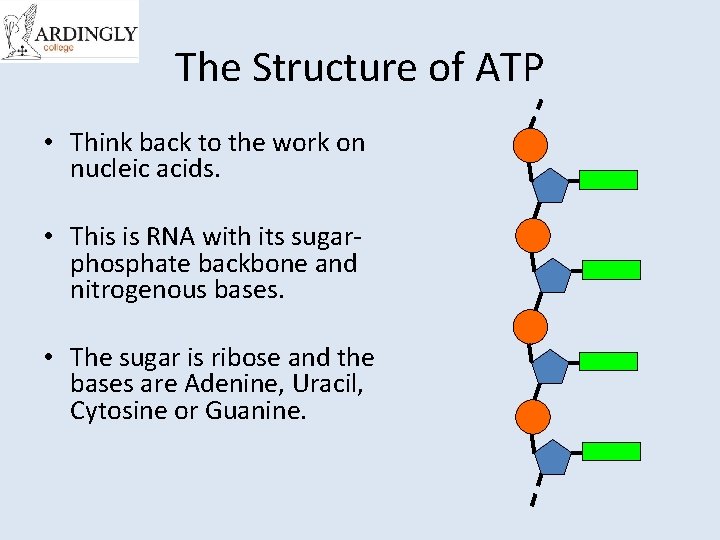
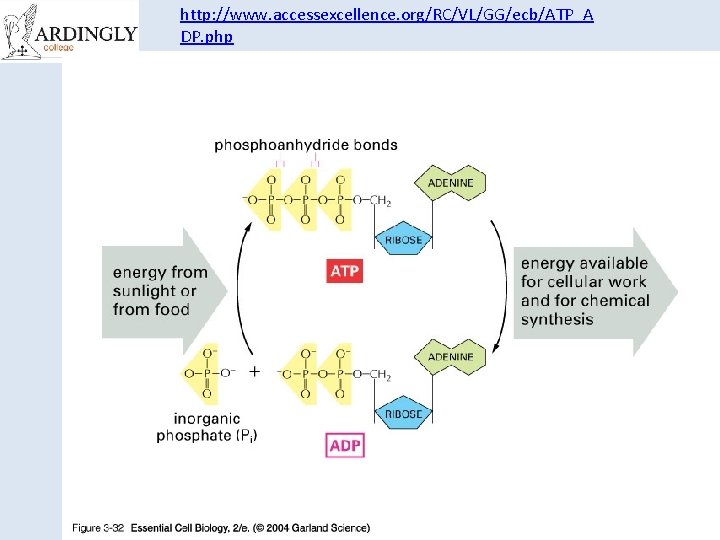
The Structure of ATP • Think back to the work on nucleic acids. • This is RNA with its sugarphosphate backbone and nitrogenous bases. • The sugar is ribose and the bases are Adenine, Uracil, Cytosine or Guanine.

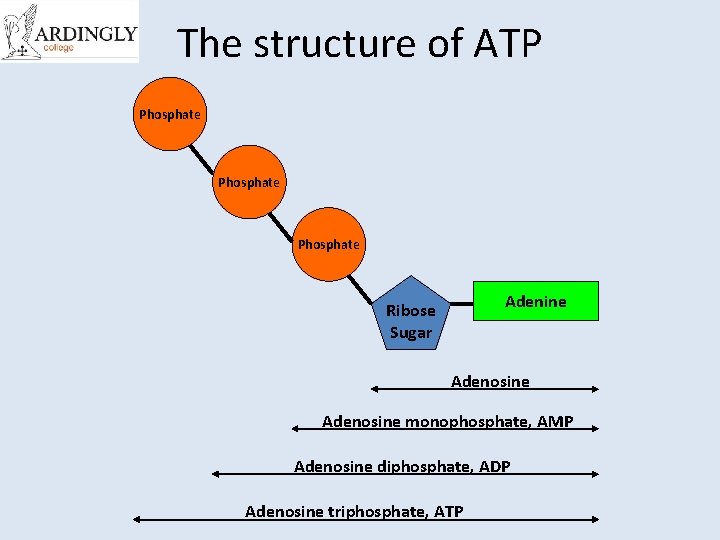
The structure of ATP Phosphate Adenine Ribose Sugar Adenosine monophosphate, AMP Adenosine diphosphate, ADP Adenosine triphosphate, ATP


http: //www. accessexcellence. org/RC/VL/GG/ecb/ATP_A DP. php

What’s a mole?

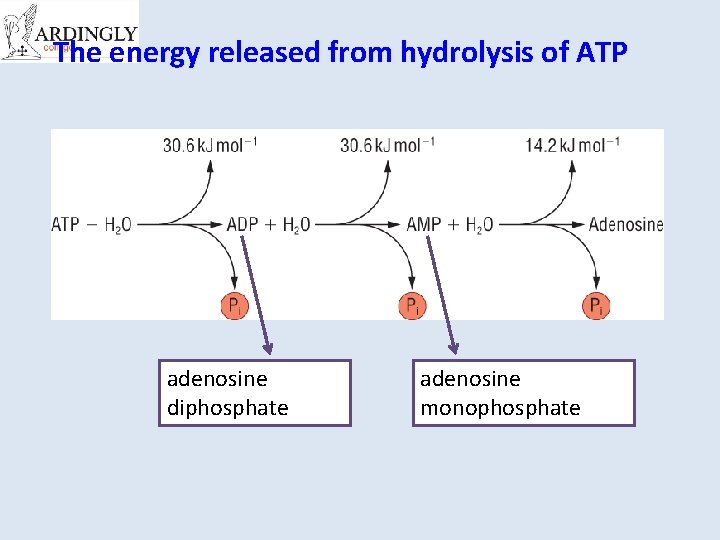
The energy released from hydrolysis of ATP adenosine diphosphate adenosine monophosphate

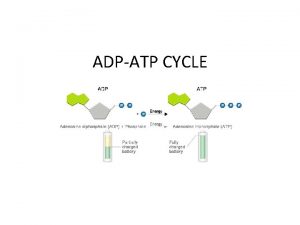
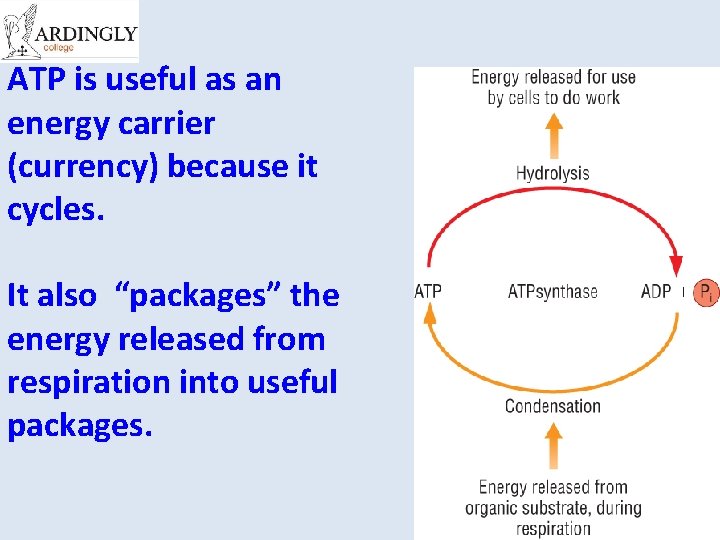
ATP is useful as an energy carrier (currency) because it cycles. It also “packages” the energy released from respiration into useful packages.

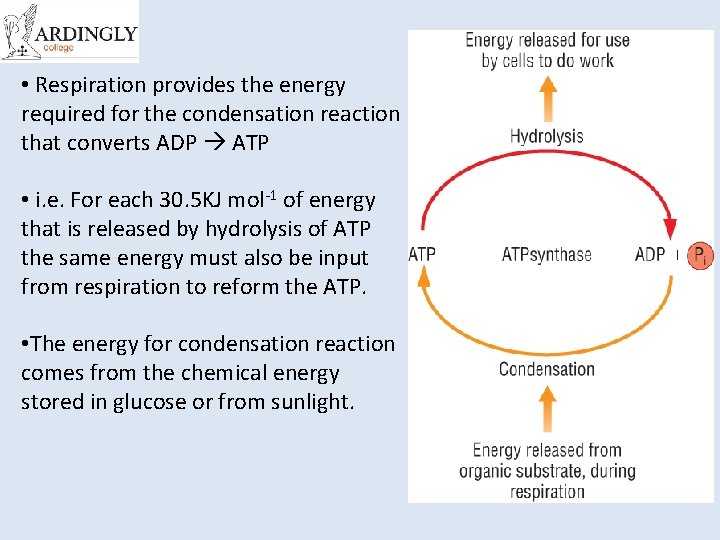
• Respiration provides the energy required for the condensation reaction that converts ADP ATP • i. e. For each 30. 5 KJ mol-1 of energy that is released by hydrolysis of ATP the same energy must also be input from respiration to reform the ATP. • The energy for condensation reaction comes from the chemical energy stored in glucose or from sunlight.

How does ATP store energy? • Each phosphate group is very negatively charged. – So they are all straining to get away from each other. – The covalent bonds holding them together are easily broken. – When they break, Pi is released along with 30. 6 k. Jmol-1 of energy for each of the first two phosphates removed. – it is literally like a loaded spring waiting to be released.

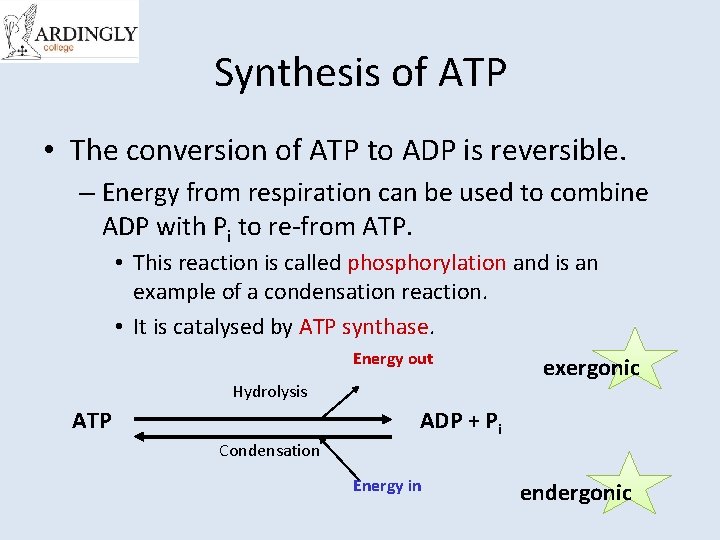
Synthesis of ATP • The conversion of ATP to ADP is reversible. – Energy from respiration can be used to combine ADP with Pi to re-from ATP. • This reaction is called phosphorylation and is an example of a condensation reaction. • It is catalysed by ATP synthase. Energy out Hydrolysis ATP exergonic ADP + Pi Condensation Energy in endergonic

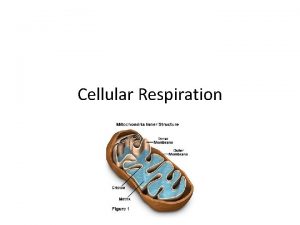
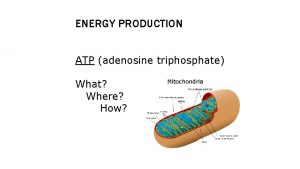
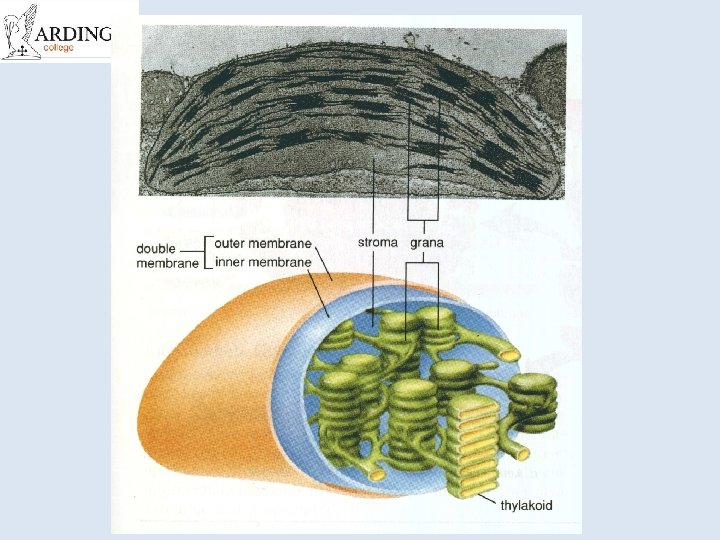
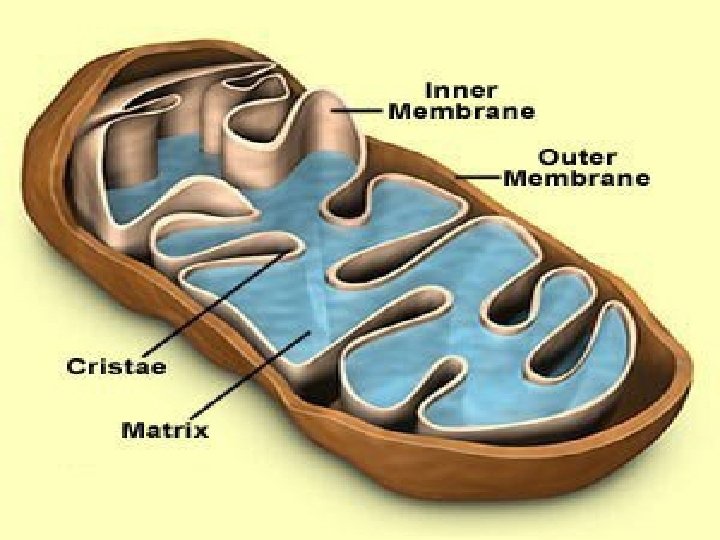
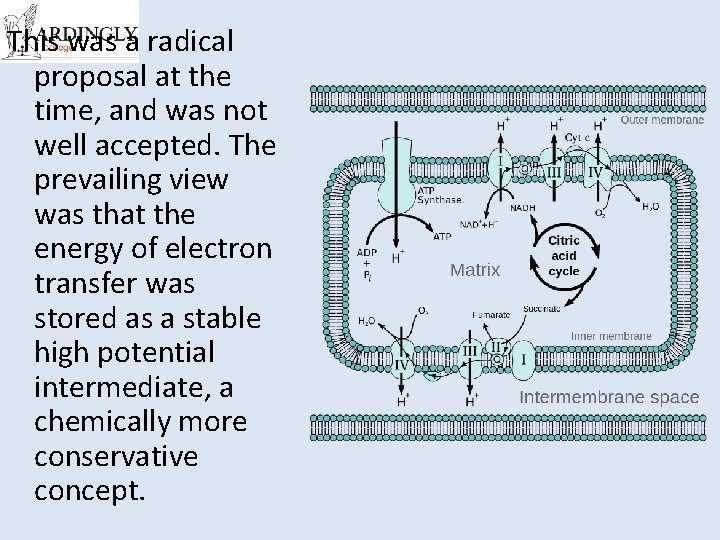
Organelles • ATP is produced in mitochondria and chloroplasts • It all happens on those internal membranes that separate: – In mitochondria, the intermembrane space from the matrix – In chloroplasts, the stroma from the thylakoid component

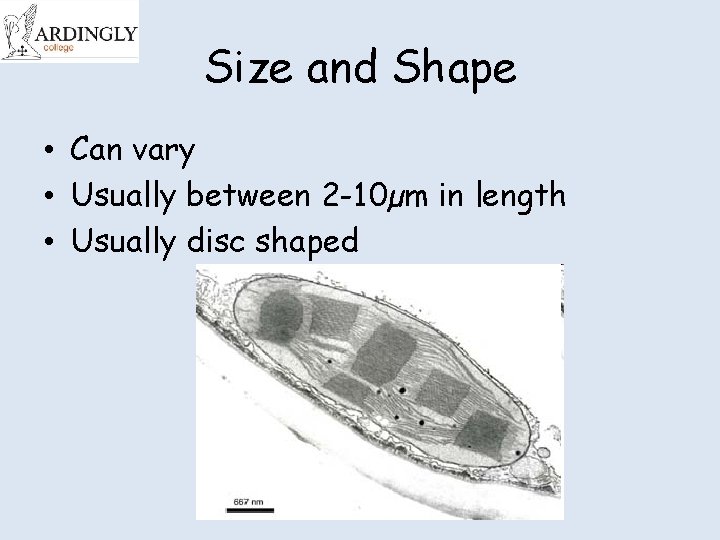
Size and Shape • Can vary • Usually between 2 -10µm in length • Usually disc shaped

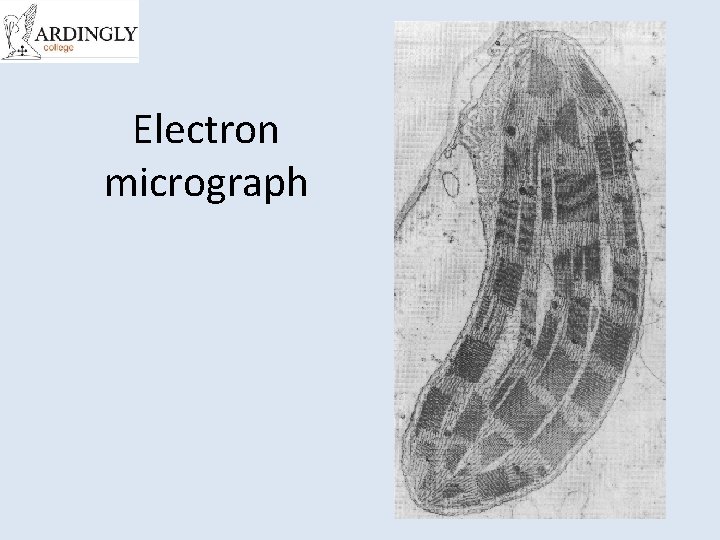
Electron micrograph



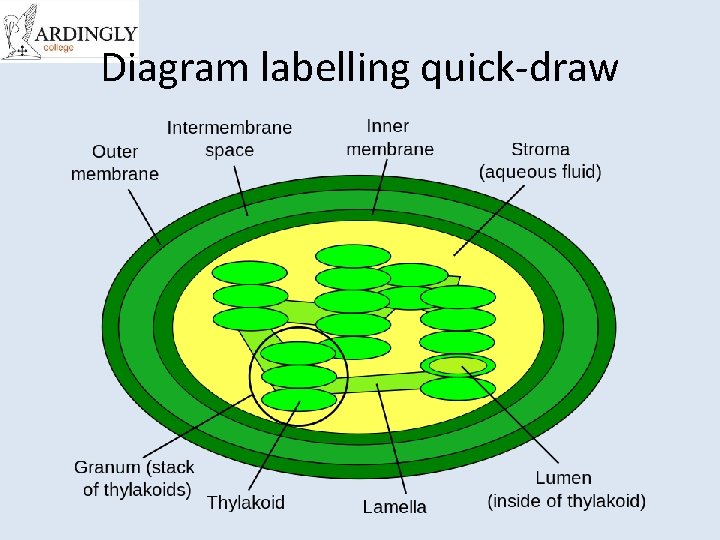
Diagram labelling quick-draw

Mitochondria

• http: //www. nobelprize. org/nobel_prizes/che mistry/laureates/1978/mitchell-speech. html


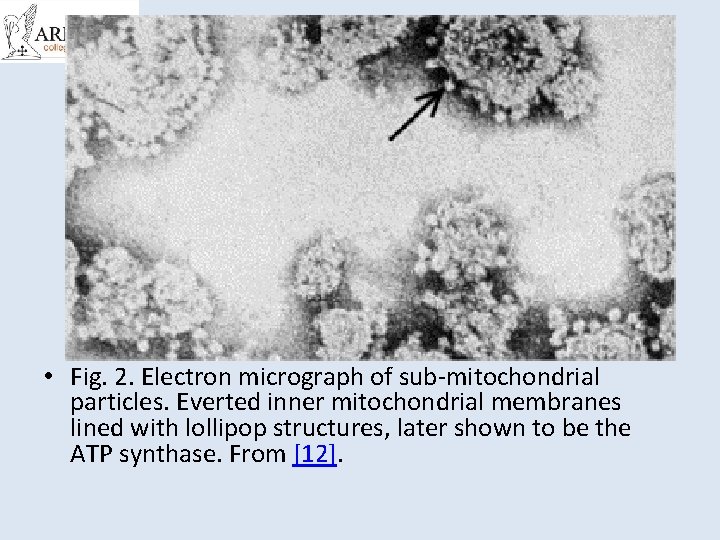
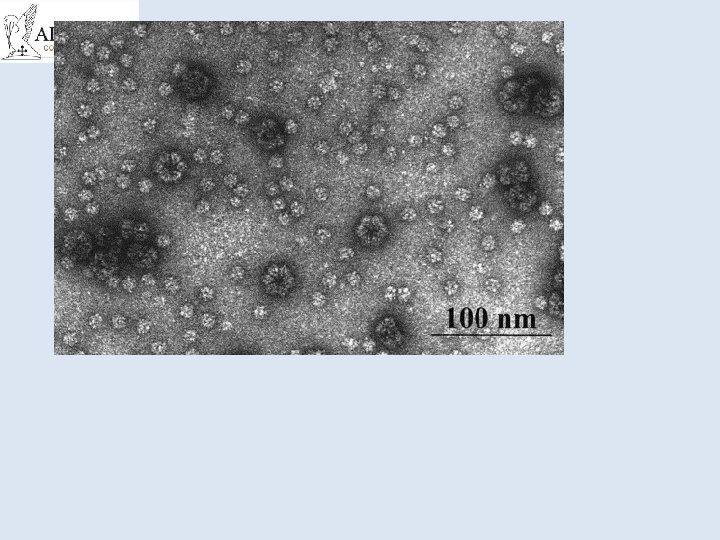
• Fig. 2. Electron micrograph of sub-mitochondrial particles. Everted inner mitochondrial membranes lined with lollipop structures, later shown to be the ATP synthase. From [12].

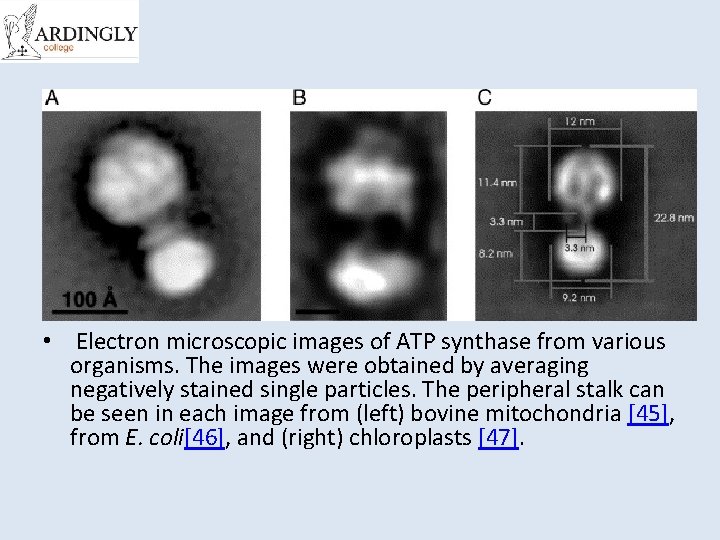
• Electron microscopic images of ATP synthase from various organisms. The images were obtained by averaging negatively stained single particles. The peripheral stalk can be seen in each image from (left) bovine mitochondria [45], from E. coli[46], and (right) chloroplasts [47].


• http: //www. sciencedirect. com/science/article /pii/S 0005272806000028

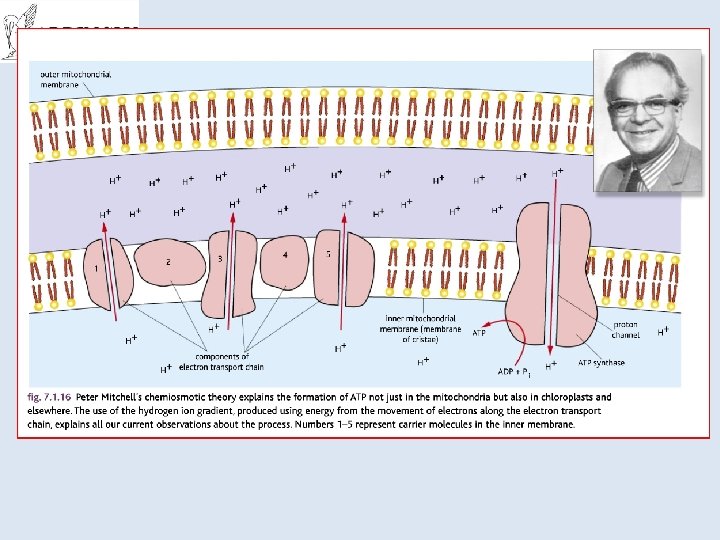
http: //www. its. caltech. edu/~sk opf/ESE_Bi 168/files/2 A. %20 Mi tchell%201961. pdf Cited by 2569!!!! Peter D. Mitchell proposed the chemiosmotic hypothesis in 1961. The theory suggests essentially that most ATP synthesis in respiring cells comes from the electrochemical gradient across the inner membranes of mitochondria by using the energy formed from the breaking down of energy-rich molecules such as glucose.

This was a radical proposal at the time, and was not well accepted. The prevailing view was that the energy of electron transfer was stored as a stable high potential intermediate, a chemically more conservative concept.

• The problem with the older paradigm is that no high energy intermediate was ever found, and the evidence for proton pumping by the complexes of the electron transfer chain grew too great to be ignored. Eventually the weight of evidence began to favour the chemiosmotic hypothesis, and in 1978, Peter Mitchell was awarded the Nobel Prize in Chemistry.

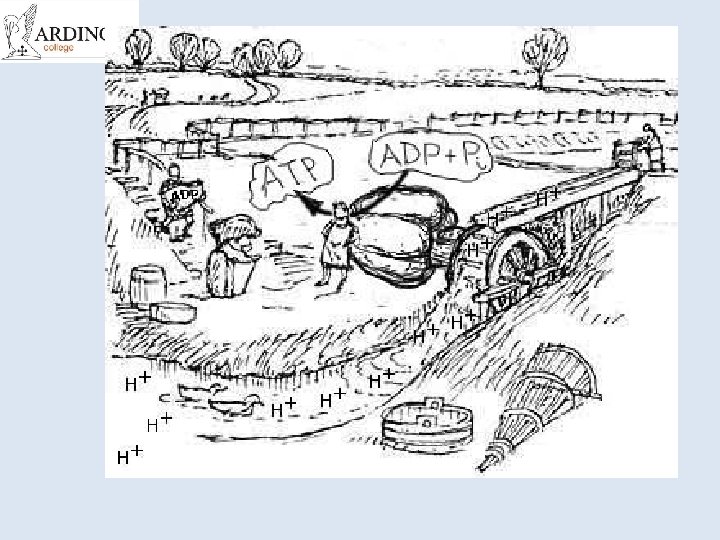
Chemiosmotic Theory of ATP production http: //www. nobelprize. org/nobel_prizes/chemistry/laureates/1978/mitchell-bio. html • Mitchell (1961) • He proposed protons are actively pumped into the inter-membrane space of the mitochondria • Done using the energy produced by electron transport chain • Results in a concentration gradient (and as they are H+ ions also a p. H difference and an electrochemical gradient)

• This means there is a tendency for the H+ ions to move back into the matrix • But they cant get across the membrane • They have to go through special pores which are linked to ATPsynthetase enzymes • The energy from the gradient drives the synthesis of ATP. • It is a universal mechanism found in all living things. • Won him the Nobel Prize in 1978


Brian Cox • https: //www. youtube. com/watch? v=qty. P 3 zk. Z Yl. E

Origin of life? • http: //www. newscientist. com/article/mg 2042 7306. 200 -was-our-oldest-ancestor-aprotonpowered-rock. html • http: //www. newscientist. com/video/4531985 5001 -lifes-origins. html

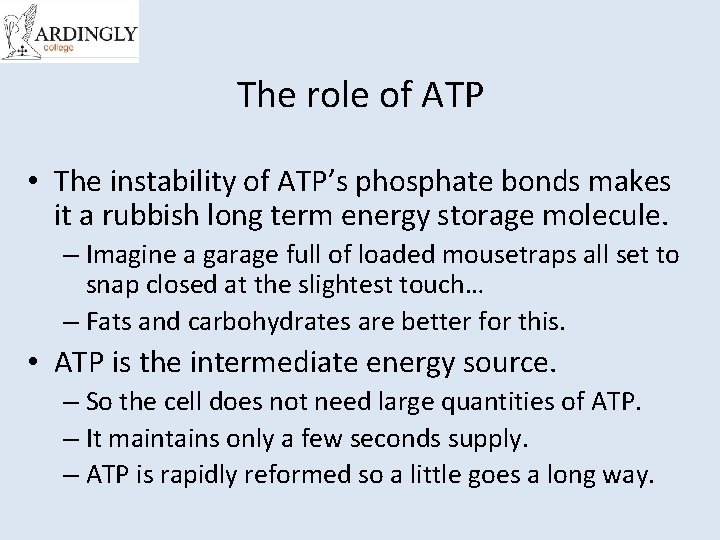
The role of ATP • The instability of ATP’s phosphate bonds makes it a rubbish long term energy storage molecule. – Imagine a garage full of loaded mousetraps all set to snap closed at the slightest touch… – Fats and carbohydrates are better for this. • ATP is the intermediate energy source. – So the cell does not need large quantities of ATP. – It maintains only a few seconds supply. – ATP is rapidly reformed so a little goes a long way.

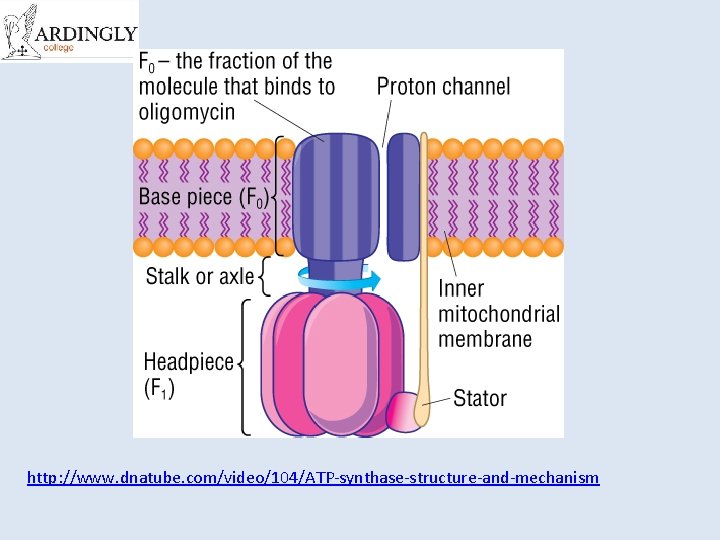
http: //www. dnatube. com/video/104/ATP-synthase-structure-and-mechanism

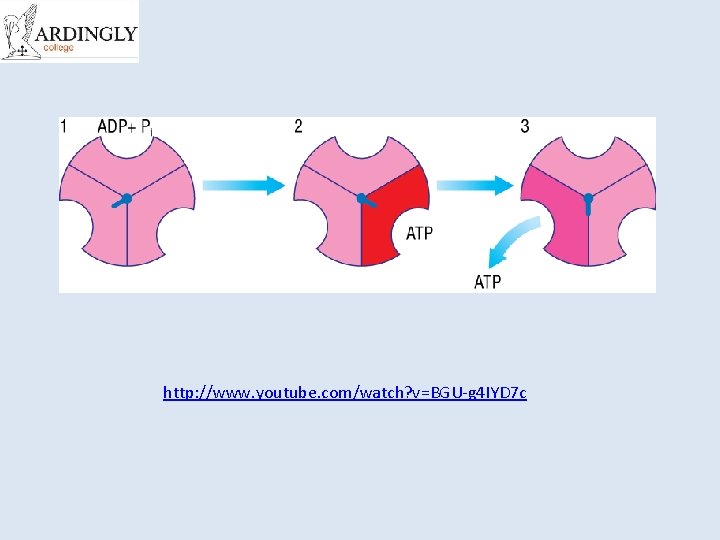
http: //www. youtube. com/watch? v=BGU-g 4 IYD 7 c

Summary • Draw a review poster to summarise the work on Energy & ATP. – Use diagrams, mind maps, flowcharts or any other method you like.
 Adenosine triphosphate (atp)
Adenosine triphosphate (atp) Kim foglia dna replication
Kim foglia dna replication Adenosine vs amiodarone
Adenosine vs amiodarone Deoxyribonucleotide adenosine
Deoxyribonucleotide adenosine Adenosine
Adenosine Factorig
Factorig Các châu lục và đại dương trên thế giới
Các châu lục và đại dương trên thế giới ưu thế lai là gì
ưu thế lai là gì Hệ hô hấp
Hệ hô hấp Các môn thể thao bắt đầu bằng từ đua
Các môn thể thao bắt đầu bằng từ đua Tư thế ngồi viết
Tư thế ngồi viết Cái miệng xinh xinh thế chỉ nói điều hay thôi
Cái miệng xinh xinh thế chỉ nói điều hay thôi Hát kết hợp bộ gõ cơ thể
Hát kết hợp bộ gõ cơ thể Cách giải mật thư tọa độ
Cách giải mật thư tọa độ Tư thế ngồi viết
Tư thế ngồi viết Thẻ vin
Thẻ vin Thế nào là giọng cùng tên
Thế nào là giọng cùng tên Chó sói
Chó sói Thơ thất ngôn tứ tuyệt đường luật
Thơ thất ngôn tứ tuyệt đường luật Các châu lục và đại dương trên thế giới
Các châu lục và đại dương trên thế giới Sự nuôi và dạy con của hươu
Sự nuôi và dạy con của hươu Từ ngữ thể hiện lòng nhân hậu
Từ ngữ thể hiện lòng nhân hậu Thế nào là hệ số cao nhất
Thế nào là hệ số cao nhất Diễn thế sinh thái là
Diễn thế sinh thái là Vẽ hình chiếu vuông góc của vật thể sau
Vẽ hình chiếu vuông góc của vật thể sau Ng-html
Ng-html 101012 bằng
101012 bằng Lời thề hippocrates
Lời thề hippocrates Tư thế worm breton là gì
Tư thế worm breton là gì đại từ thay thế
đại từ thay thế Quá trình desamine hóa có thể tạo ra
Quá trình desamine hóa có thể tạo ra Công thức tính độ biến thiên đông lượng
Công thức tính độ biến thiên đông lượng Thế nào là mạng điện lắp đặt kiểu nổi
Thế nào là mạng điện lắp đặt kiểu nổi Khi nào hổ mẹ dạy hổ con săn mồi
Khi nào hổ mẹ dạy hổ con săn mồi Các loại đột biến cấu trúc nhiễm sắc thể
Các loại đột biến cấu trúc nhiễm sắc thể Nguyên nhân của sự mỏi cơ sinh 8
Nguyên nhân của sự mỏi cơ sinh 8 Bổ thể
Bổ thể Vẽ hình chiếu đứng bằng cạnh của vật thể
Vẽ hình chiếu đứng bằng cạnh của vật thể độ dài liên kết
độ dài liên kết Thiếu nhi thế giới liên hoan
Thiếu nhi thế giới liên hoan Bài hát chúa yêu trần thế alleluia
Bài hát chúa yêu trần thế alleluia điện thế nghỉ
điện thế nghỉ Một số thể thơ truyền thống
Một số thể thơ truyền thống Trời xanh đây là của chúng ta thể thơ
Trời xanh đây là của chúng ta thể thơ