4 1 Representing Ionic Compounds Prayer Lord you













- Slides: 18

4. 1 Representing Ionic Compounds

Prayer Lord, you said that when two or three would gather together in your name, then you would be present with them. I am praying by myself (or ‘on the Internet’) but I am uniting myself with many individual Christians throughout the world who, though separate, are gathered together in another sense to pray to you, and I trust that you are with me now.

Forming Ionic Compounds Elements can combine to make ionic compounds Ionic Compound: A compound composed of oppositely charged ions; a metal and a non-metal.

Cations & Anions & Charges When an atom loses or gains electron and becomes charged, it becomes an ion When an atom loses an electron, it becomes positive When an atom gains an electron, it becomes negative Positively Charged Ion = Cation Negatively Charged Ion= Anion

When electrons are TRANSFERRED from one atom to another, an Ionic Bond is taking place

Properties of Ionic Compounds State Conductivity Structure Melting points Solids Yes; in liquid! Crystal structure (Salt) Very high Boiling points Very high

Valence Electrons There are patterns related to the arrangement of electrons in atoms in the periodic table. Elements in the same Groups have the same number of valence electrons Valence Electrons: electrons in the outer energy level These are involved in chemical bonding between elements

Valence Electrons It helps to know the number of valence electrons that each group has so that you can: Predict formation of compounds Name compounds Write chemical formulas

Naming Binary Compounds 2 ways to identify a compound: Chemical Name Chemical Formula

RULES: First identify the Positive Ion (the metal cation) Second part of the name always identifies the Negative Ion (non-metal anion) Change the ending of the Negative Ion/Non-metal anion to –ide Chemical Names Manesium & Phosphorus Magnesium Phosphide Sodium & Chlorine Sodium Chloride Calcium & Bromine Calcium Bromide Aluminum & Oxygen Aluminum Oxide

Chemical Formula Metal Non-metal Charges? Flip-flop! If the charge is 1? Drop it.

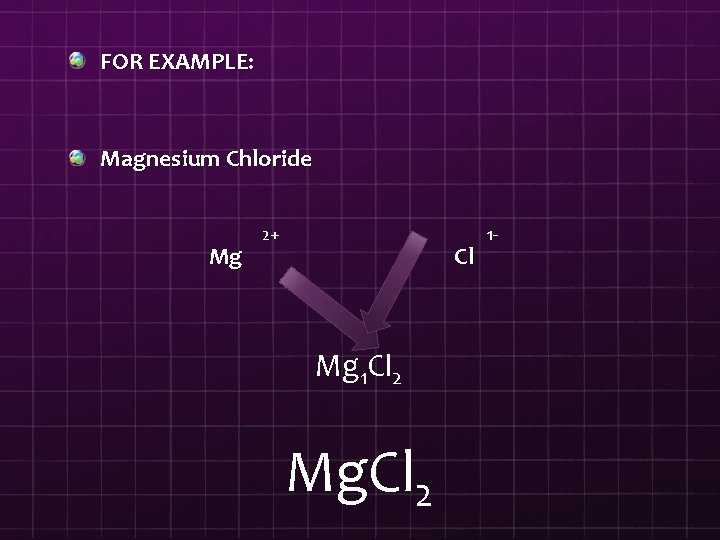
FOR EXAMPLE: Magnesium Chloride Mg 2+ Cl Mg 1 Cl 2 Mg. Cl 2 1 -

Multivalent Metals Check out your periodic table; you may notice some elements have more than one charge! These are called MULTIVALENT METALS Copper, for example, can form ions with either a charge of +1 or +2.

In your booklet: Stock System vs. Classical System of naming Ie. Copper (II) Sulfide. OR Cupric Sulfide Check the charges, refer to the BACK of your pink periodic table.

Writing Chemical Formulas and Naming Ionic Compounds with a Multivalent Metal When naming a compound that has a multivalent ion, you must include a Roman numeral to indicate which charge the ion has. Ie. Copper (II) Sulfide: Cu. S

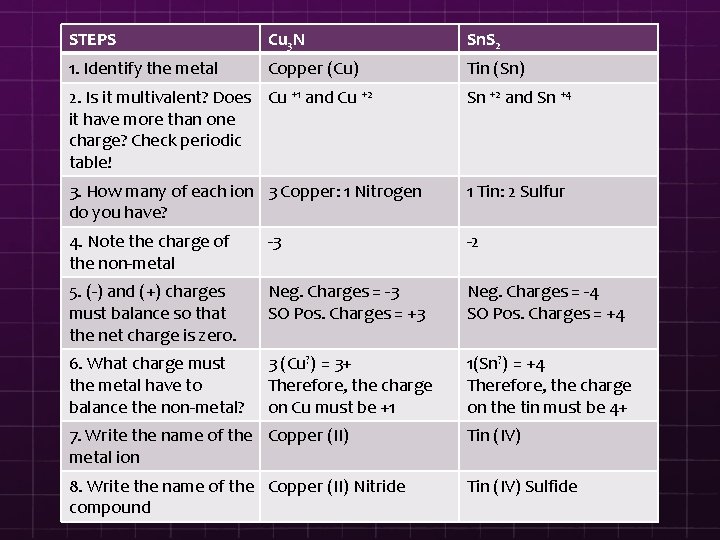
STEPS Cu 3 N Sn. S 2 1. Identify the metal Copper (Cu) Tin (Sn) 2. Is it multivalent? Does Cu +1 and Cu +2 it have more than one charge? Check periodic table! Sn +2 and Sn +4 3. How many of each ion 3 Copper: 1 Nitrogen do you have? 1 Tin: 2 Sulfur 4. Note the charge of the non-metal -3 -2 5. (-) and (+) charges must balance so that the net charge is zero. Neg. Charges = -3 SO Pos. Charges = +3 Neg. Charges = -4 SO Pos. Charges = +4 6. What charge must the metal have to balance the non-metal? 3 (Cu? ) = 3+ Therefore, the charge on Cu must be +1 1(Sn? ) = +4 Therefore, the charge on the tin must be 4+ 7. Write the name of the Copper (II) metal ion Tin (IV) 8. Write the name of the Copper (II) Nitride compound Tin (IV) Sulfide

Example: Sn 3 P 4 STEPS 1. Identify the metal 1. Tin 2. Is it multivalent? Does it have more than one charge? Check periodic table! 2. Yes: Sn +2 and Sn+4 3. How many of each ion do you have? 4. -3 4. Note the charge of the non-metal 5. Total Negative Charge: -12 5. (-) and (+) charges must balance so that the net charge is zero. 6. What charge must the metal have to balance the non-metal? 7. Write the name of the metal ion 8. Write the name of the compound 3. 3 Tin: 4 Phosphorous SO the Total Pos. Charge = +12 6. 3 (Sn? ) = 12+ Therefore the charge of tin must be 4. 7. Tin (IV) 8. Tin (IV) Phosphide

Sn 3 P 4 You could also do the reverse cross-over method: Sn 3 P 4 Sn 4 P 3 (Sn +4) (P -3)