4 03 Atomic Structure Nomenclature and Parts of

- Slides: 34

4. 03 Atomic Structure Nomenclature and Parts of the atom Dr. Fred Omega Garces Chemistry 100 Miramar College 1 4. 03 Atomic Structure August 2017

Modern view of the atom What are the parts of the atom and how are these parts (especially the electrons) arranged in each atom? What makes each atom (element) different from each other ? What makes certain atoms (elements) similar to each other ? 2 4. 03 Atomic Structure August 2017

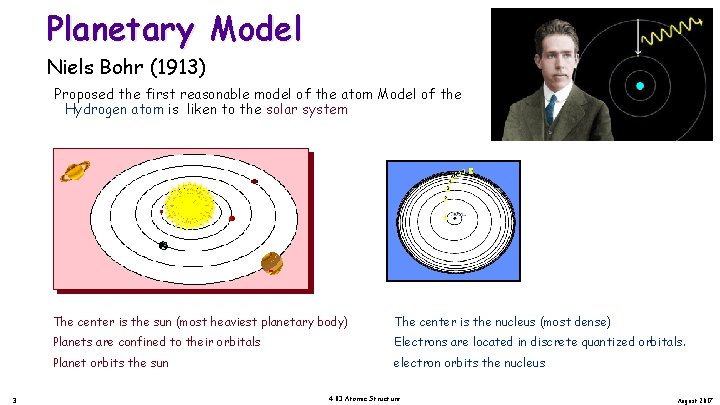
Planetary Model Niels Bohr (1913) Proposed the first reasonable model of the atom Model of the Hydrogen atom is liken to the solar system 3 The center is the sun (most heaviest planetary body) The center is the nucleus (most dense) Planets are confined to their orbitals Electrons are located in discrete quantized orbitals. Planet orbits the sun electron orbits the nucleus 4. 03 Atomic Structure August 2017

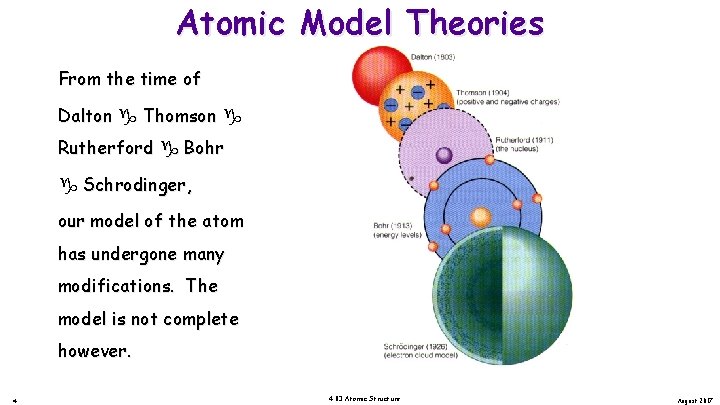
Atomic Model Theories From the time of Dalton Thomson Rutherford Bohr Schrodinger, our model of the atom has undergone many modifications. The model is not complete however. 4 4. 03 Atomic Structure August 2017

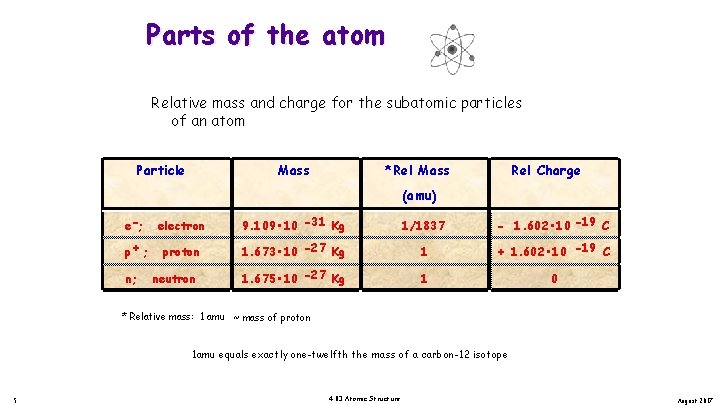
Parts of the atom Relative mass and charge for the subatomic particles of an atom Particle Mass *Rel Mass Rel Charge (amu) e -; electron 9. 109 • 10 -31 Kg 1/1837 - 1. 602 • 10 -19 C p+; proton 1. 673 • 10 -27 Kg 1 + 1. 602 • 10 -19 C 1. 675 • 10 -27 Kg 1 0 n; neutron * Relative mass: 1 amu ~ mass of proton 1 amu equals exactly one-twelfth the mass of a carbon-12 isotope 5 4. 03 Atomic Structure August 2017

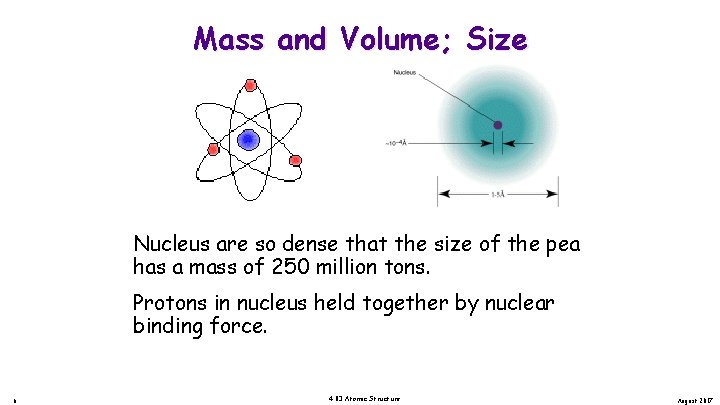
Mass and Volume; Size Nucleus are so dense that the size of the pea has a mass of 250 million tons. Protons in nucleus held together by nuclear binding force. 6 4. 03 Atomic Structure August 2017

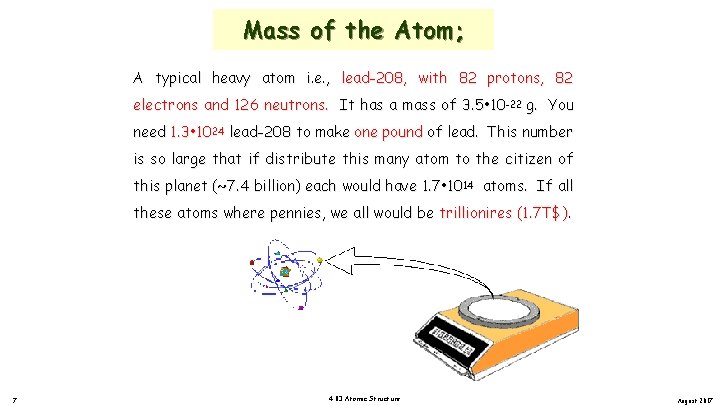
Mass of the Atom; A typical heavy atom i. e. , lead-208, with 82 protons, 82 electrons and 126 neutrons. It has a mass of 3. 5 • 10 -22 g. You need 1. 3 • 1024 lead-208 to make one pound of lead. This number is so large that if distribute this many atom to the citizen of this planet (~7. 4 billion) each would have 1. 7 • 1014 atoms. If all these atoms where pennies, we all would be trillionires (1. 7 T$). 7 4. 03 Atomic Structure August 2017

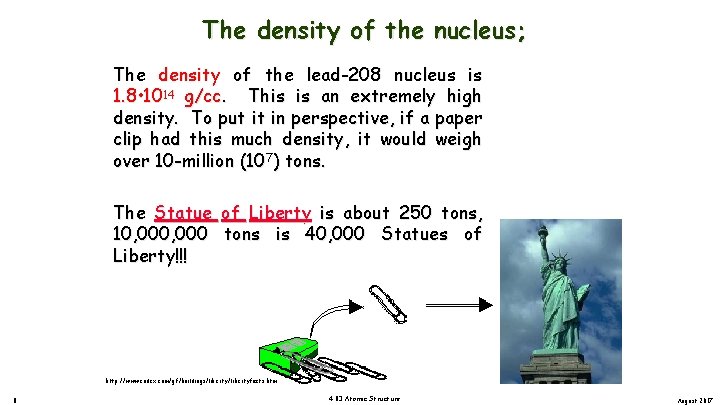
The density of the nucleus; The density of the lead-208 nucleus is 1. 8 • 1014 g/cc. This is an extremely high density. To put it in perspective, if a paper clip had this much density, it would weigh over 10 -million (107) tons. The Statue of Liberty is about 250 tons, 10, 000 tons is 40, 000 Statues of Liberty!!! http: //www. endex. com/gf/buildings/libertyfacts. htm 8 4. 03 Atomic Structure August 2017

The size of the nucleus; If the nucleus of a lead-208 atom were the size of a golf ball, then the whole atom would be much larger than a Coliseum stadium (LA coliseum). In fact, it would be a sphere about half-mile in diameter. 9 4. 03 Atomic Structure August 2017

Mass of the electron; An electron has a mass of 9. 1 • 10 -28 g. The sun is very big, it weigh about 333, 000 times as much as the earth. The mass of the sun is to the mass of the pineapple as the mass of the pineapple is to the mass of an electron. Electron 10 4. 03 Atomic Structure August 2017

Mass of the nucleus; The total mass of the 82 electrons in a lead-208 atom is 7. 5 • 10 -26 g, the mass of the whole atom is only 3. 5 • 10 -22 g ( the mass only accounts for 1/4500 of its mass). An single electron 1/2000 th the mass of a penny. The mass of a nickel is about 5 g. If I had 35 nickel in my pocket, then my 154 -lb (70 kg) is to weight of a proton as the 35 nickels is to weigh of an electron. 11 4. 03 Atomic Structure August 2017

Size of the Atom; An atom has a diameter of about 3. 5 • 10 -8 cm. If you could line them up with the atoms just touching, it would take 73 million (73, 000) atoms to make a line 1 inch long. 12 4. 03 Atomic Structure August 2017

Why are elements so different ? (if particles are identical) Electrons dictate chemistry, it is the electron which interact with each other. Their arrangement is different for each element which give rise to the element’s different properties. 13 4. 03 Atomic Structure August 2017

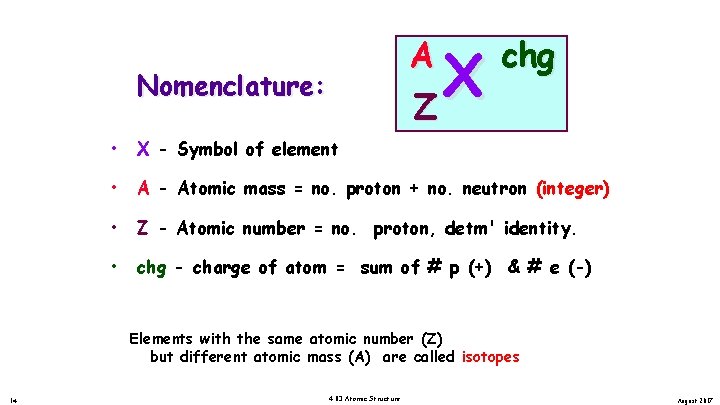
A Z Nomenclature: X chg • X - Symbol of element • A - Atomic mass = no. proton + no. neutron (integer) • Z - Atomic number = no. proton, detm' identity. • chg - charge of atom = sum of # p (+) & # e (-) Elements with the same atomic number (Z) but different atomic mass (A) are called isotopes 14 4. 03 Atomic Structure August 2017

The atoms: Different Elements The periodic table give an atomic mass that is not a whole number? i. e. , C = 12. 011 amu, H = 1. 008 amu, O = 15. 9994 amu 15 4. 03 Atomic Structure August 2017

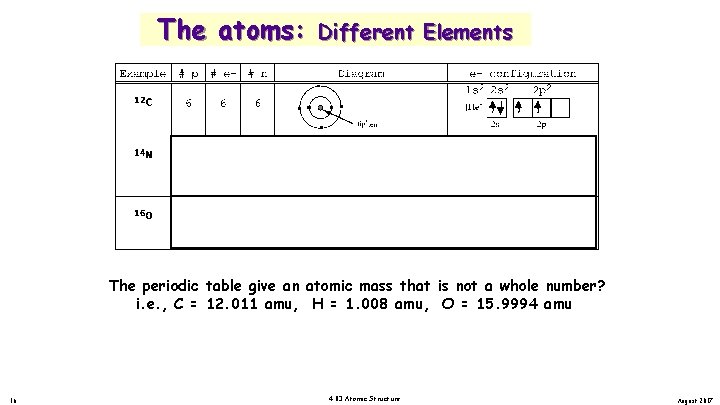
The atoms: Different Elements The periodic table give an atomic mass that is not a whole number? i. e. , C = 12. 011 amu, H = 1. 008 amu, O = 15. 9994 amu 16 4. 03 Atomic Structure August 2017

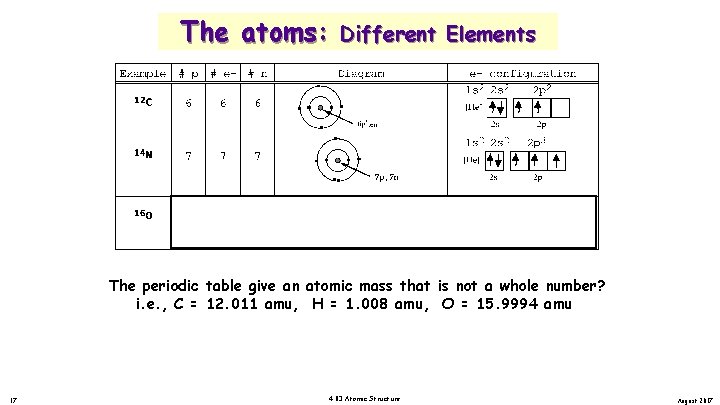
The atoms: Different Elements The periodic table give an atomic mass that is not a whole number? i. e. , C = 12. 011 amu, H = 1. 008 amu, O = 15. 9994 amu 17 4. 03 Atomic Structure August 2017

The atoms: Different Elements The periodic table give an atomic mass that is not a whole number? i. e. , C = 12. 011 amu, H = 1. 008 amu, O = 15. 9994 amu 18 4. 03 Atomic Structure August 2017

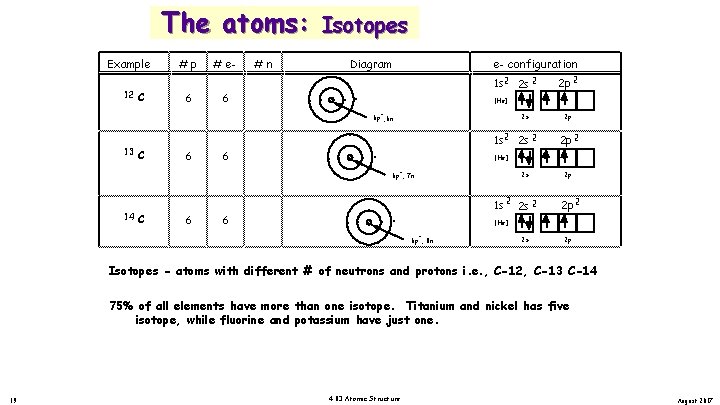
The atoms: Example 12 C #p 6 # e 6 #n 6 Isotopes. Diagram . . . . e- configuration 1 s 2 2 s 2 [He] + 2 s 6 p , 6 n 13 C 6 6 7 1 s 2 2 s 2 C 6 6 8 2 p 2 p 2 [He] + 2 s 6 p , 7 n 14 2 p 2 1 s 2 2 p 2 p 2 [He] + 6 p , 8 n 2 s 2 p Isotopes - atoms with different # of neutrons and protons i. e. , C-12, C-13 C-14 75% of all elements have more than one isotope. Titanium and nickel has five isotope, while fluorine and potassium have just one. 19 4. 03 Atomic Structure August 2017

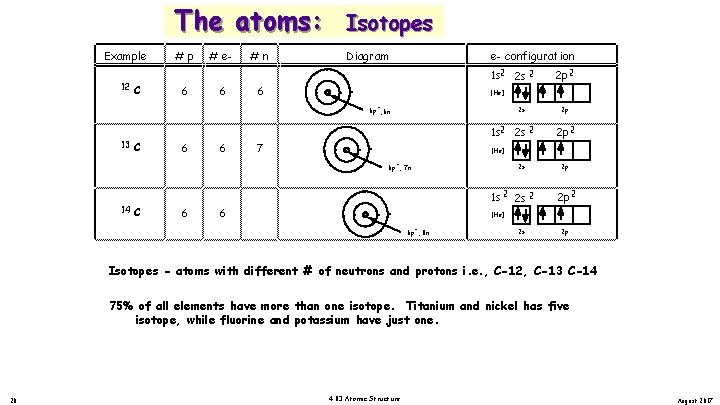
Example 12 C The atoms: Isotopes #p Diagram 6 # e 6 #n 6 . . . . e- configuration 1 s 2 2 s 2 [He] + 2 s 6 p , 6 n 13 C 6 6 7 1 s 2 2 s 2 C 6 6 8 2 p 2 p 2 [He] + 2 s 6 p , 7 n 14 2 p 2 1 s 2 2 p 2 p 2 [He] + 6 p , 8 n 2 s 2 p Isotopes - atoms with different # of neutrons and protons i. e. , C-12, C-13 C-14 75% of all elements have more than one isotope. Titanium and nickel has five isotope, while fluorine and potassium have just one. 20 4. 03 Atomic Structure August 2017

The atoms: Example 12 C #p 6 # e 6 #n 6 . Isotopes Diagram . . . . e- configuration 1 s 2 2 s 2 [He] + 2 s 6 p , 6 n 13 C 6 6 7 1 s 2 2 s 2 C 6 6 8 2 p 2 p 2 [He] + 2 s 6 p , 7 n 14 2 p 2 1 s 2 2 p 2 p 2 [He] + 6 p , 8 n 2 s 2 p Isotopes - atoms with different # of neutrons and protons i. e. , C-12, C-13 C-14 75% of all elements have more than one isotope. Titanium and nickel has five isotope, while fluorine and potassium have just one. 21 4. 03 Atomic Structure August 2017

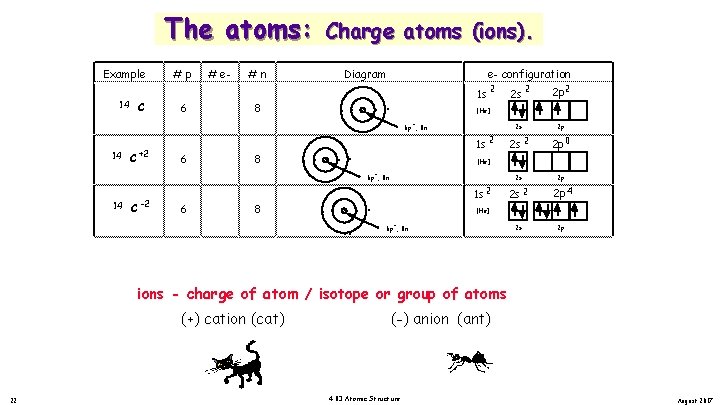
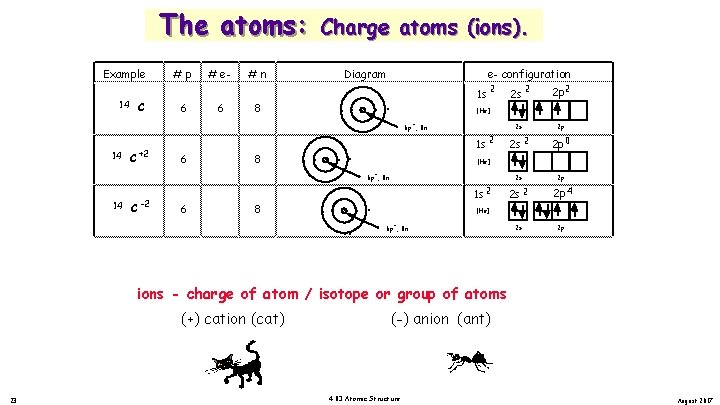
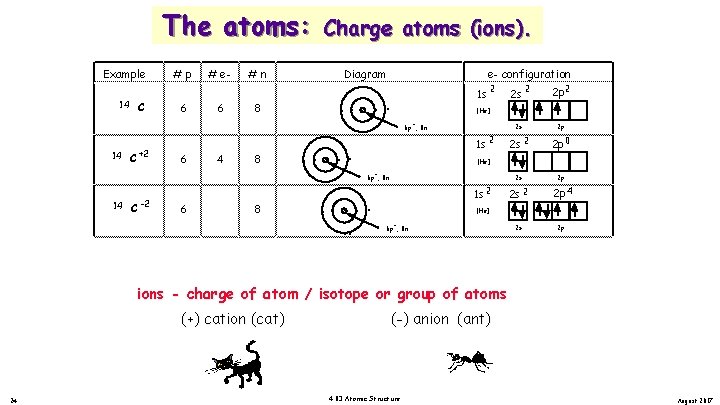
The atoms: Example 14 14 C +2 C #p 6 6 # e 6 4 #n 8 8 Charge atoms (ions). Diagram e- configuration . . . . 2 1 s 6 p , 8 n 1 s 2 C 6 8 8 2 p 2 2 s 2 p 2 s 2 2 p 0 2 s 2 p 2 s 2 2 p 4 2 s 2 p [He] 6 p , 8 n 14 2 [He] + + -2 2 s 1 s 2 [He] + 6 p , 8 n ions - charge of atom / isotope or group of atoms (+) cation (cat) 22 (-) anion (ant) 4. 03 Atomic Structure August 2017

The atoms: Example 14 14 C +2 C #p 6 6 # e 6 4 #n 8 8 Charge atoms (ions). Diagram e- configuration . . . . 2 1 s 6 p , 8 n 1 s 2 C 6 8 8 2 p 2 2 s 2 p 2 s 2 2 p 0 2 s 2 p 2 s 2 2 p 4 2 s 2 p [He] 6 p , 8 n 14 2 [He] + + -2 2 s 1 s 2 [He] + 6 p , 8 n ions - charge of atom / isotope or group of atoms (+) cation (cat) 23 (-) anion (ant) 4. 03 Atomic Structure August 2017

The atoms: Example 14 14 C +2 C #p 6 6 # e 6 4 #n 8 8 Charge atoms (ions). Diagram e- configuration . . . . 2 1 s 6 p , 8 n 1 s 2 C 6 8 8 2 p 2 2 s 2 p 2 s 2 2 p 0 2 s 2 p 2 s 2 2 p 4 2 s 2 p [He] 6 p , 8 n 14 2 [He] + + -2 2 s 1 s 2 [He] + 6 p , 8 n ions - charge of atom / isotope or group of atoms (+) cation (cat) 24 (-) anion (ant) 4. 03 Atomic Structure August 2017

The atoms: Example 14 14 C +2 C #p 6 6 # e 6 4 #n 8 8 Charge atoms (ions). Diagram e- configuration . . . . 2 1 s 6 p , 8 n 1 s 2 C 6 8 8 2 p 2 2 s 2 p 2 s 2 2 p 0 2 s 2 p 2 s 2 2 p 4 2 s 2 p [He] 6 p , 8 n 14 2 [He] + + -2 2 s 1 s 2 [He] + 6 p , 8 n ions - charge of atom / isotope or group of atoms (+) cation (cat) 25 (-) anion (ant) 4. 03 Atomic Structure August 2017

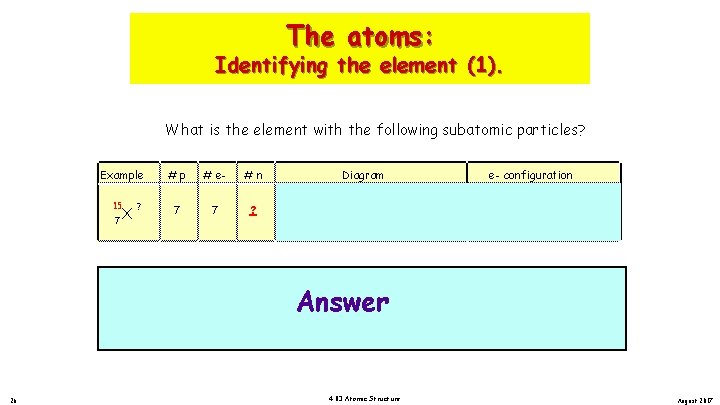
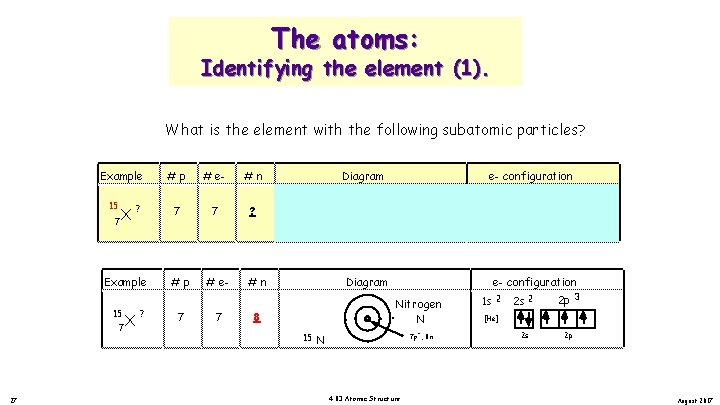
The atoms: Identifying the element (1). What is the element with the following subatomic particles? Example 15 X 7 ? ? 26 X 7 # e 7 #n Diagram ? 15 Example 7 #p ? #p 7 # e 7 #n 7 N . . Nitrogen. . N. e- configuration 1 s 2 2 s 7 p , 7 n . . Nitrogen Answer. . N 14 N. + 7 p , 7 n 4. 03 Atomic Structure 2 p 3 [He] + Diagram 2 s 2 2 p e- configuration 1 s 2 2 p 3 [He] 2 s 2 p August 2017

The atoms: Identifying the element (1). What is the element with the following subatomic particles? Example 15 X 7 ? 7 27 X 7 # e 7 #n Diagram ? 14 Example 15 #p ? #p 7 # e 7 N #n . . Nitrogen. . N. 15 N 1 s 2 2 s + . . Nitrogen. . N. + 7 p , 8 n 4. 03 Atomic Structure 2 s 2 2 p 3 [He] 7 p , 7 n Diagram 8 e- configuration 2 p e- configuration 1 s 2 2 p 3 [He] 2 s 2 p August 2017

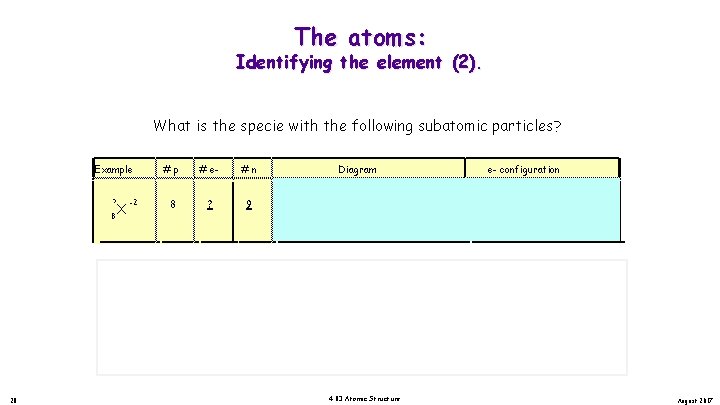
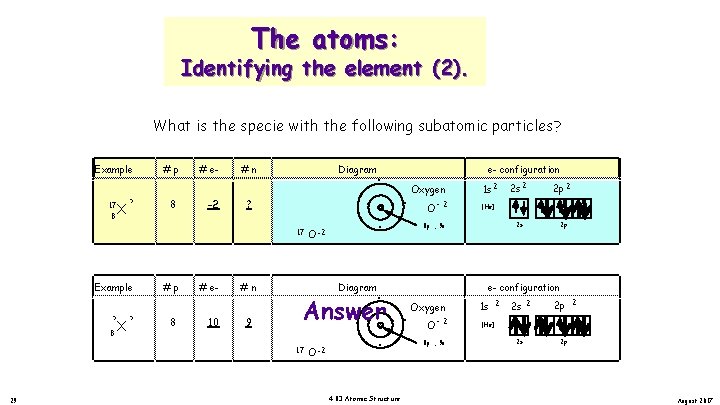
The atoms: Identifying the element (2). What is the specie with the following subatomic particles? Example ? 8 X #p -2 8 # e? #n Diagram . . . 9 17 Example 17 8 X -2 #p 8 # e 10 O -2 #n . . . Oxygen O -2 9 . . Oxygen. . . O - 2. . 4. 03 Atomic Structure 8 p , 9 n 2 s 2 2 p 2 2 s 2 p [He] 8 p , 9 n + O -2 1 s 2 + Diagram 17 28 e- configuration 1 s 2 2 p 2 [He] 2 s 2 p August 2017

The atoms: Identifying the element (2). What is the specie with the following subatomic particles? Example 17 8 X ? #p 8 # e-2 #n Diagram . . . ? 17 Example ? X 8 ? #p 8 # e 10 #n 9 . . . O -2 Oxygen Answer. . O -2 . 4. 03 Atomic Structure + 8 p , 9 n 2 s 2 2 p 2 2 s 2 p [He] 8 p , 9 n . O -2 1 s 2 + Diagram 17 29 e- configuration 1 s 2 2 p 2 [He] 2 s 2 p August 2017

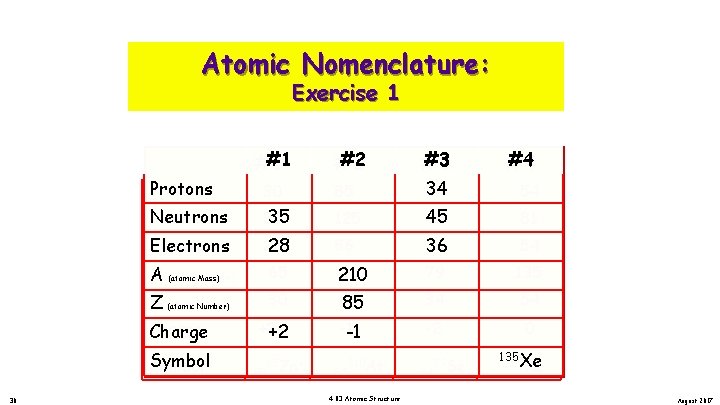
Atomic Nomenclature: Exercise 1 Protons Neutrons Electrons A (atomic Mass) A Mass) Z (atomic No. ) Z (atomic Number) Charge Symbol 30 #1 #1 #1 #2 #2 #2 30 85 35 35 35 125 28 28 28 65 30 +2 +2 +2 30 65 Zn+2 86 210 113 85 85 48 -1 -1 +2 85 210 At- 4. 03 Atomic Structure #3 #3 #3 34 34 33 45 45 44 36 36 36 #4 #4 #4 54 81 54 79 135 34 54 -2 0 34 79 Se-2 135 222 135 Xe Rn Xe August 2017

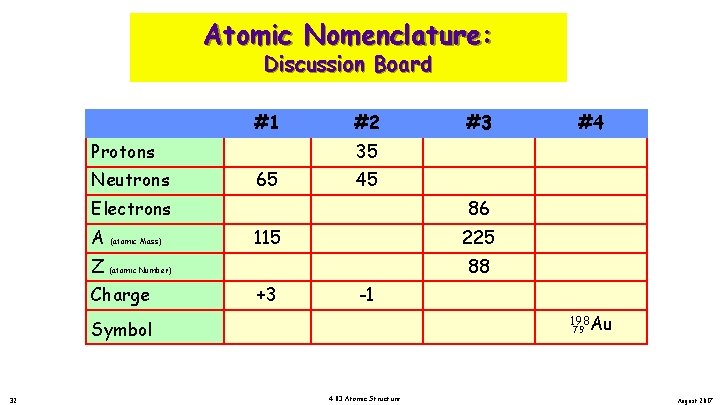
Atomic Nomenclature: Discussion Board #1 Protons #2 65 45 Electrons A (atomic Mass) 86 115 225 Z (atomic Number) Charge 32 #4 35 Neutrons Symbol #3 88 +3 -1 . 198 Au 79 4. 03 Atomic Structure August 2017

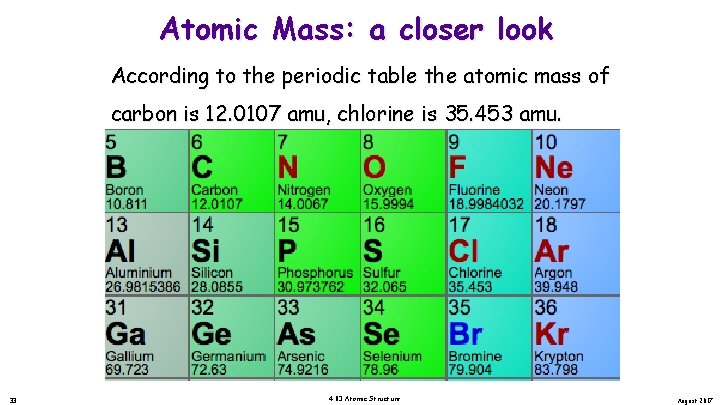
Atomic Mass: a closer look According to the periodic table the atomic mass of carbon is 12. 0107 amu, chlorine is 35. 453 amu. Yet we just said that the mass of an atom is arrived by the number of protons and neutrons for that atom. How is it that we have 0. 11 amu worth of atom. Is there such a thing as a fraction of an atom? 33 4. 03 Atomic Structure August 2017

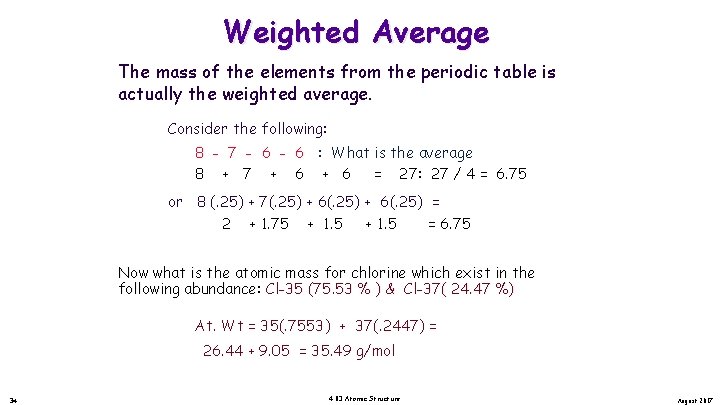
Weighted Average The mass of the elements from the periodic table is actually the weighted average. Consider the following: 8 - 7 - 6 : What is the average 8 + 7 + 6 = 27: 27 / 4 = 6. 75 or 8 (. 25) + 7(. 25) + 6(. 25) = 2 + 1. 75 + 1. 5 = 6. 75 Now what is the atomic mass for chlorine which exist in the following abundance: Cl-35 (75. 53 % ) & Cl-37( 24. 47 %) At. Wt = 35(. 7553) + 37(. 2447) = 26. 44 + 9. 05 = 35. 49 g/mol 34 4. 03 Atomic Structure August 2017

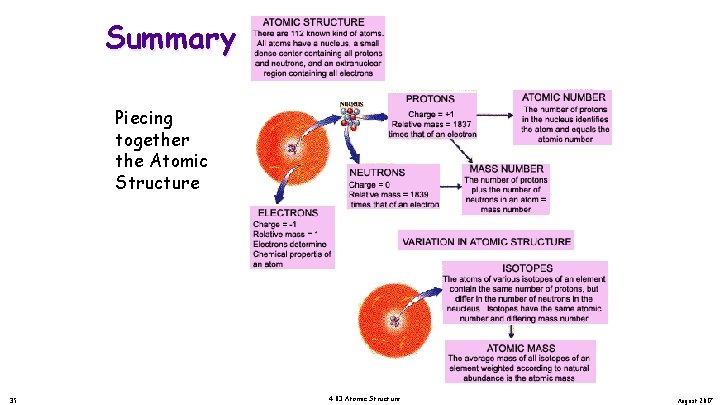
Summary Piecing together the Atomic Structure 35 4. 03 Atomic Structure August 2017