300 0 400 20 10 500 30 600















- Slides: 56

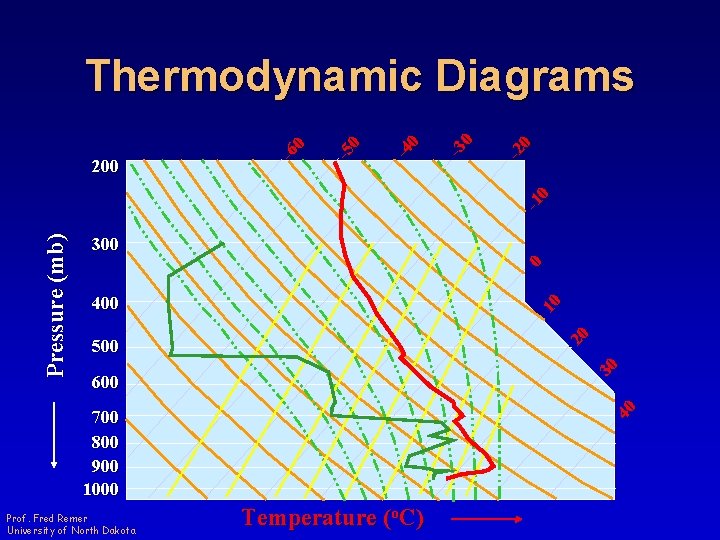
300 0 400 20 10 500 30 600 40 Pressure (mb) -1 0 -2 0 -3 0 -4 0 -5 0 200 -6 0 Thermodynamic Diagrams 700 800 900 1000 Prof. Fred Remer University of North Dakota Temperature (o. C)

Thermodynamic Diagrams • Reading – Hess • Chapter 5 – pp 65 – 74 – Tsonis • pp 143 – 150 – Air Weather Service, AWS/TR-79/006 – Wallace & Hobbs • pp 78 – 79 Prof. Fred Remer University of North Dakota

Thermodynamic Diagrams • Objectives – Be able to list the three desirable characteristics of a thermodynamic diagram – Be able to describe how a transformation is made from p, a coordinates when designing a thermodynamic diagram Prof. Fred Remer University of North Dakota

Thermodynamic Diagrams • Objectives – Be able to list the coordinates of each thermodynamic diagram – Be able to describe the advantages and disadvantages of each thermodynamic diagram Prof. Fred Remer University of North Dakota

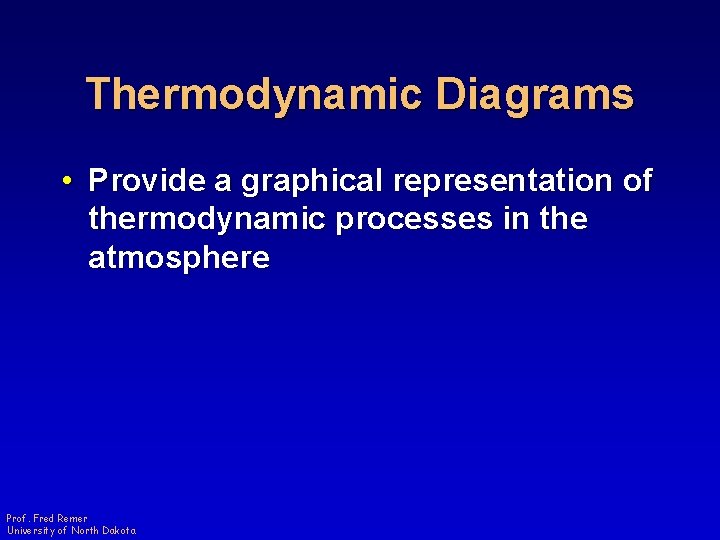
Thermodynamic Diagrams • Provide a graphical representation of thermodynamic processes in the atmosphere Prof. Fred Remer University of North Dakota

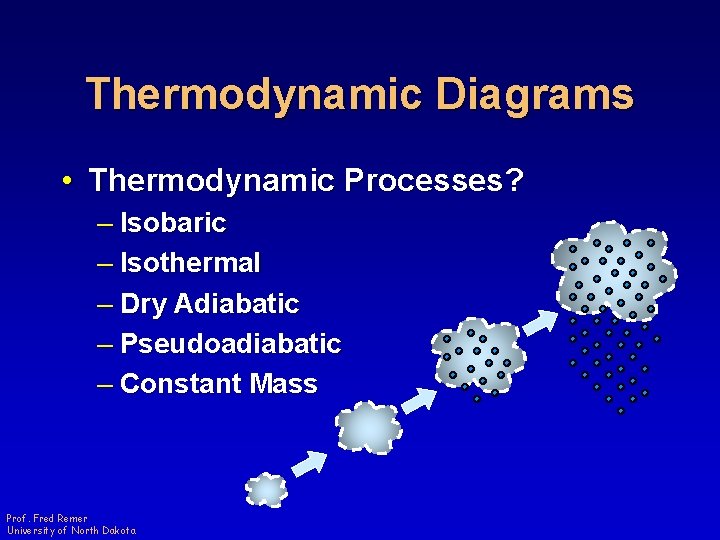
Thermodynamic Diagrams • Thermodynamic Processes? – Isobaric – Isothermal – Dry Adiabatic – Pseudoadiabatic – Constant Mass Prof. Fred Remer University of North Dakota

Thermodynamic Diagrams • Thermodynamic Diagrams – Eliminates or simplifies calculations Prof. Fred Remer University of North Dakota

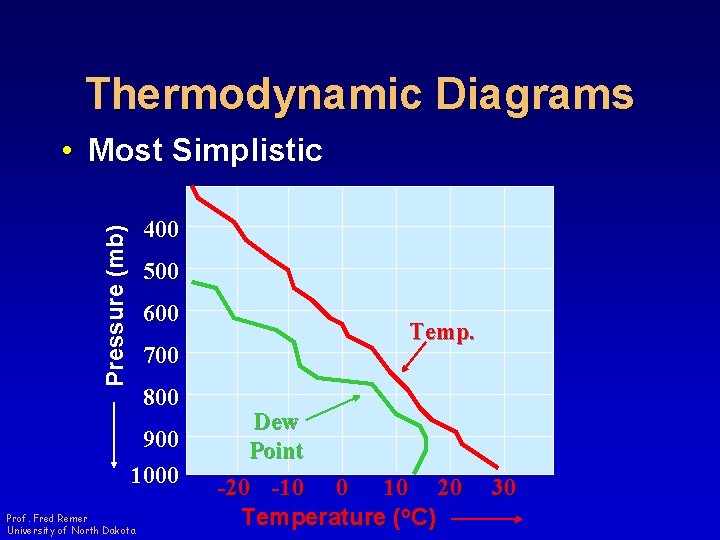
Thermodynamic Diagrams • Most Simplistic Pressure (mb) 400 500 600 Temp. 700 800 900 1000 Prof. Fred Remer University of North Dakota Dew Point -20 -10 0 10 20 Temperature (o. C) 30

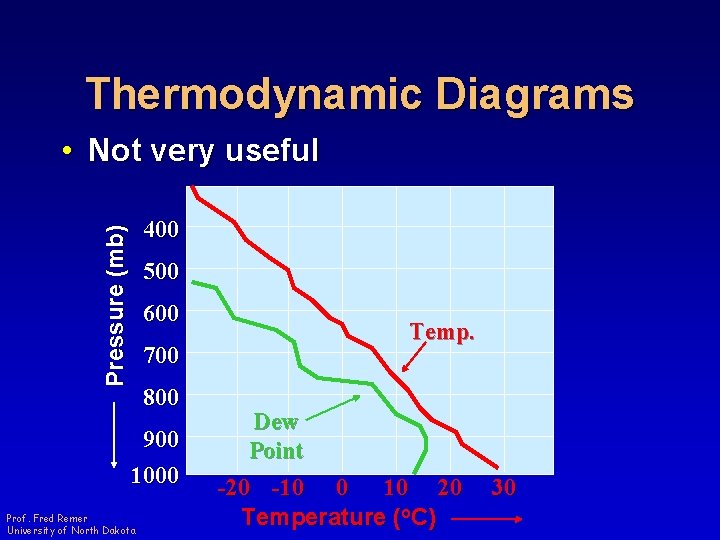
Thermodynamic Diagrams • Not very useful Pressure (mb) 400 500 600 Temp. 700 800 900 1000 Prof. Fred Remer University of North Dakota Dew Point -20 -10 0 10 20 Temperature (o. C) 30

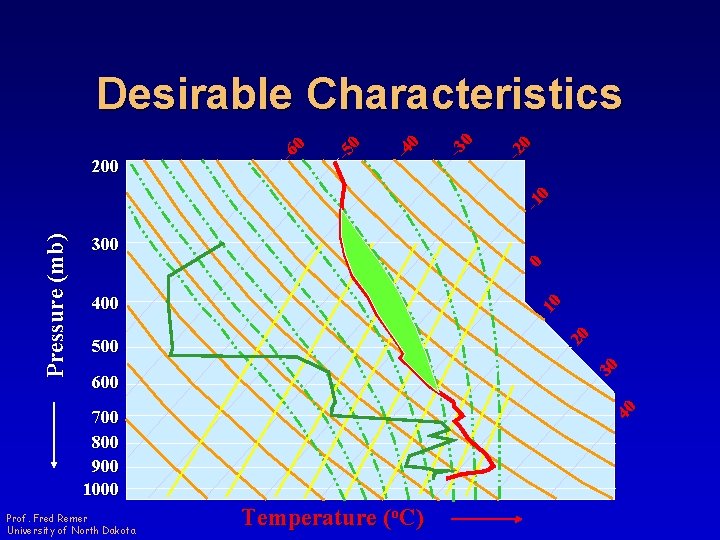
Thermodynamic Diagrams • Desirable Characteristics – Area Equivalent • Area enclosed by a cyclic process is proportional to energy Prof. Fred Remer University of North Dakota

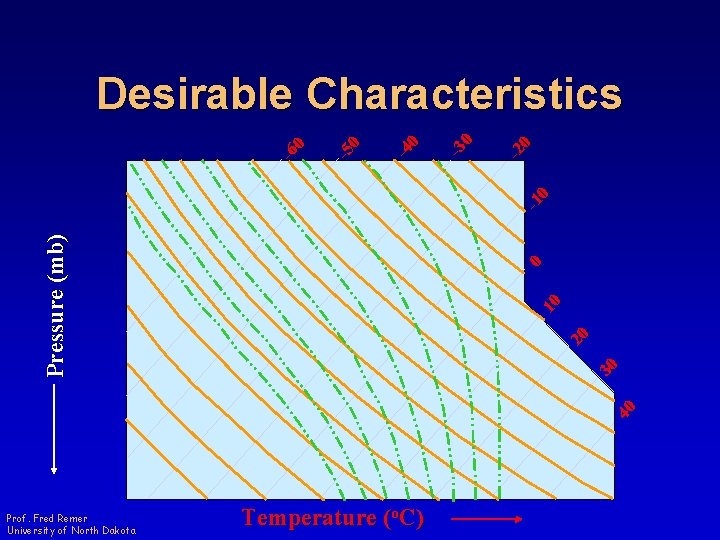
300 0 400 20 10 500 30 600 40 Pressure (mb) -1 0 -2 0 -3 0 -4 0 -5 0 200 -6 0 Desirable Characteristics 700 800 900 1000 Prof. Fred Remer University of North Dakota Temperature (o. C)

Desirable Characteristics • As many isopleths as possible be straight lines Prof. Fred Remer University of North Dakota

300 0 400 20 10 500 30 600 40 Pressure (mb) -1 0 -2 0 -3 0 -4 0 -5 0 200 -6 0 Desirable Characteristics 700 800 900 1000 Prof. Fred Remer University of North Dakota Temperature (o. C)

Desirable Characteristics • The angle between isotherms and adiabats be as large as possible – Sensitivity to the rate of change of temperature with pressure in the vertical • Easier to determine stability of the environment – 90 o Optimum Prof. Fred Remer University of North Dakota

40 30 20 10 0 Pressure (mb) -1 0 -2 0 -3 0 -4 0 -5 0 -6 0 Desirable Characteristics Prof. Fred Remer University of North Dakota Temperature (o. C)

Coordinates • Select so that it satisfies Area Equivalent characteristic – Enclosed area is proportional to energy • Use p & a Prof. Fred Remer University of North Dakota

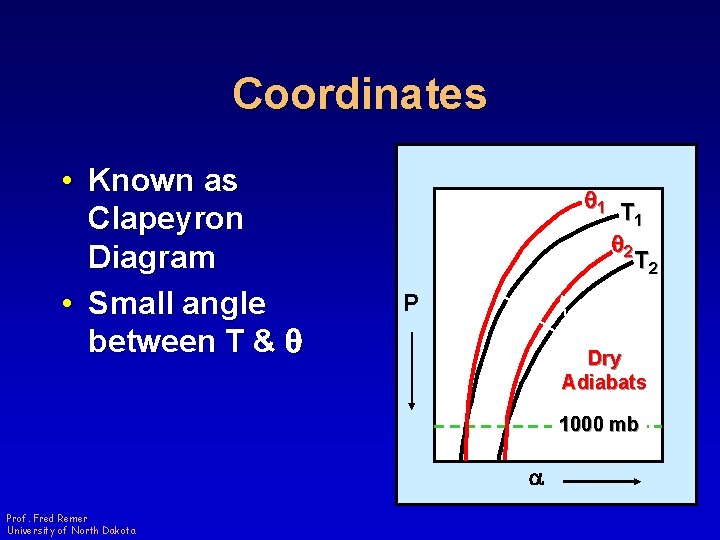
Coordinates • Known as Clapeyron Diagram • Small angle between T & q q 1 T 1 q 2 T 2 P Dry Adiabats 1000 mb a Prof. Fred Remer University of North Dakota

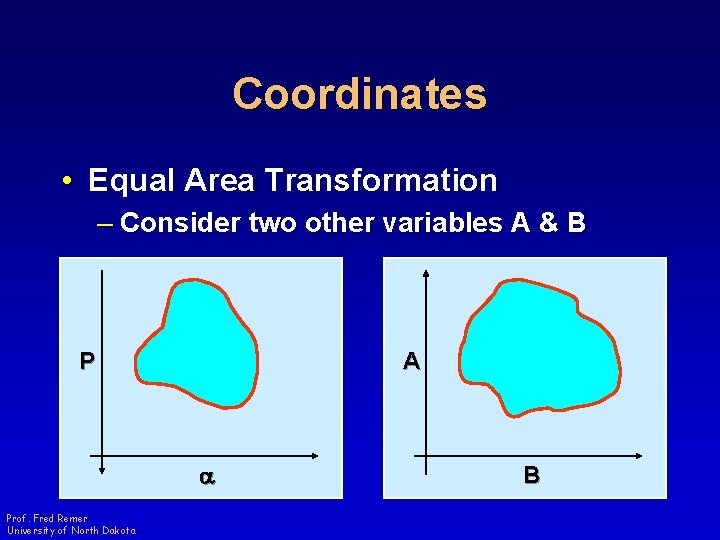
Coordinates • Equal Area Transformation – Consider two other variables A & B P A a Prof. Fred Remer University of North Dakota B

Coordinates • Equal Area Transformation – Create a transformation from -p, a to A, B P A a Prof. Fred Remer University of North Dakota B

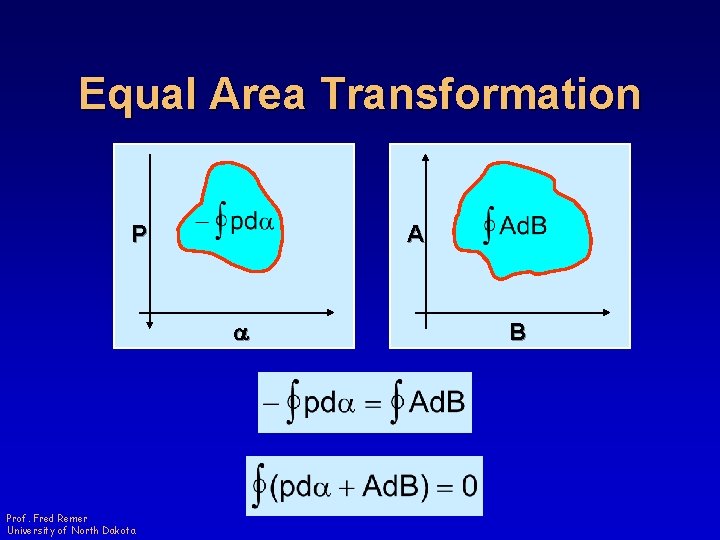
Equal Area Transformation P A a Prof. Fred Remer University of North Dakota B

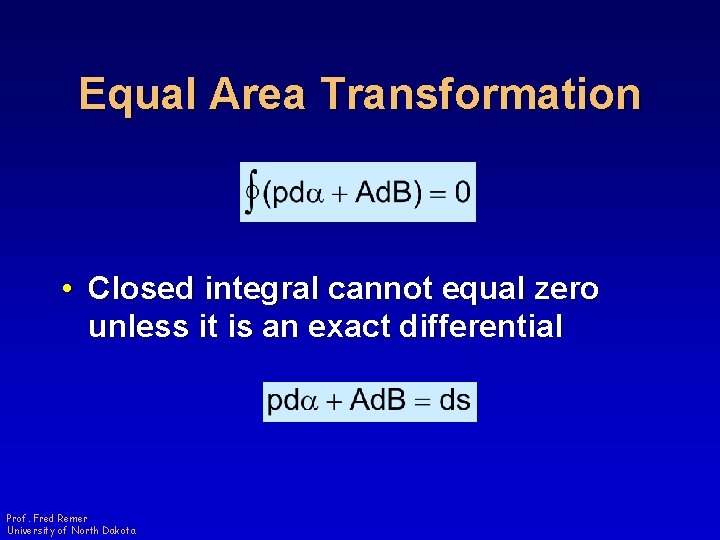
Equal Area Transformation • Closed integral cannot equal zero unless it is an exact differential Prof. Fred Remer University of North Dakota

Equal Area Transformation • Differentiate s with respect to a and B • So. . . Prof. Fred Remer University of North Dakota

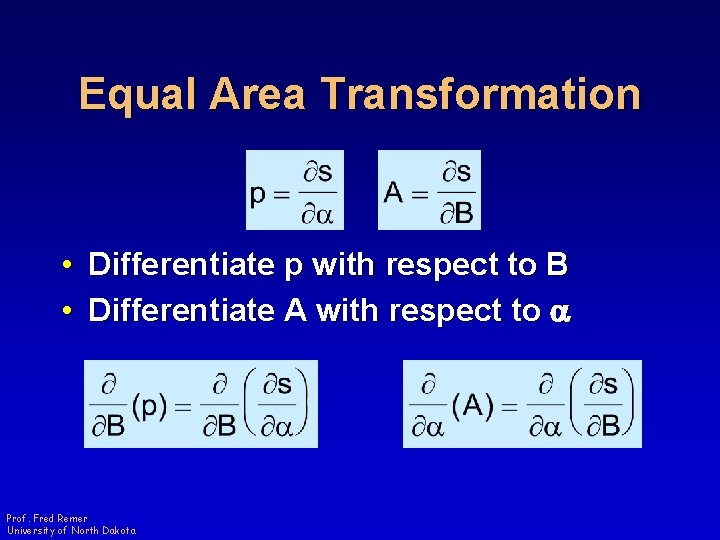
Equal Area Transformation • Differentiate p with respect to B • Differentiate A with respect to a Prof. Fred Remer University of North Dakota

Equal Area Transformation • So… Prof. Fred Remer University of North Dakota

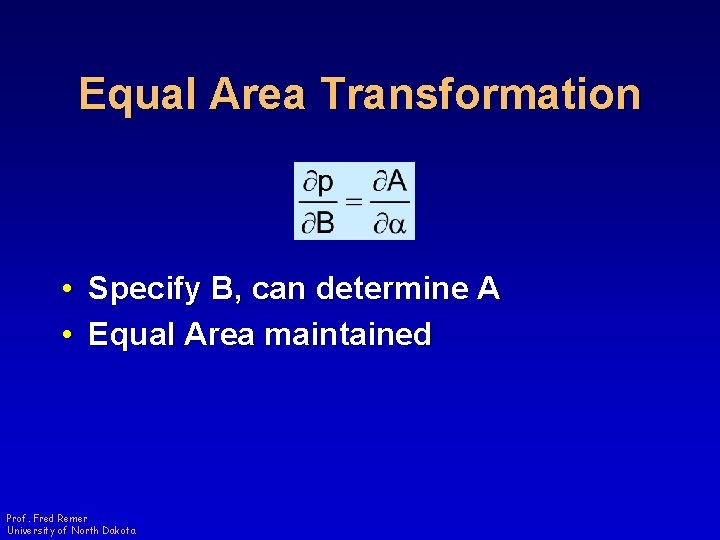
Equal Area Transformation • Specify B, can determine A • Equal Area maintained Prof. Fred Remer University of North Dakota

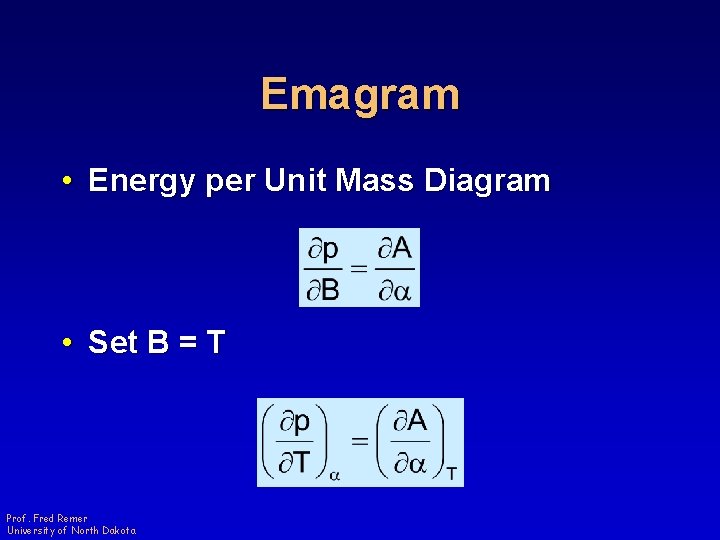
Emagram • Energy per Unit Mass Diagram • Set B = T Prof. Fred Remer University of North Dakota

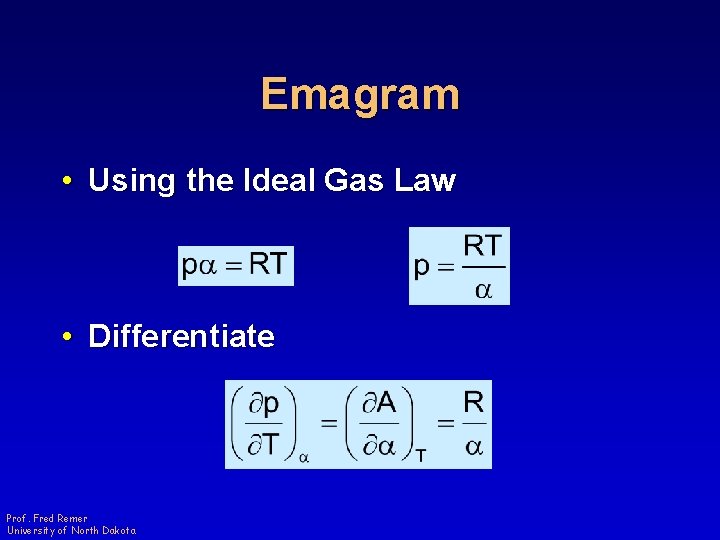
Emagram • Using the Ideal Gas Law • Differentiate Prof. Fred Remer University of North Dakota

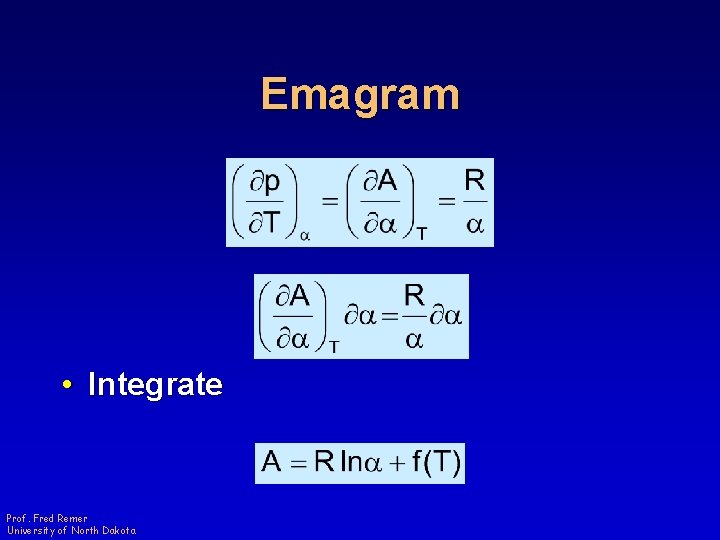
Emagram • Integrate Prof. Fred Remer University of North Dakota

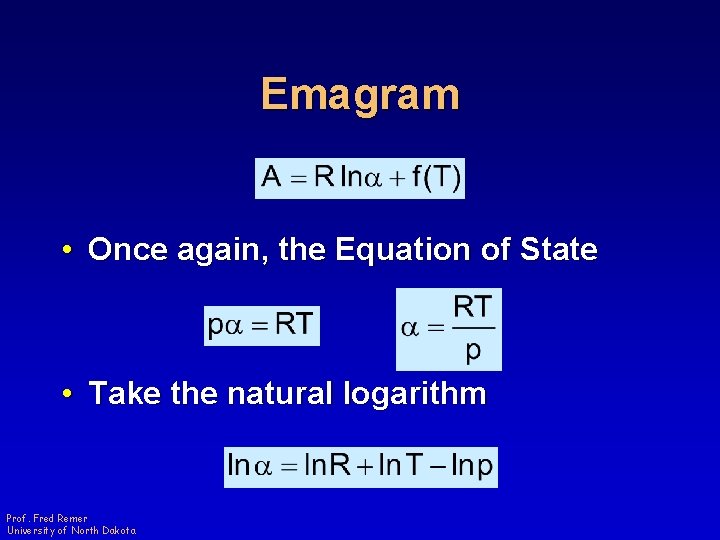
Emagram • Once again, the Equation of State • Take the natural logarithm Prof. Fred Remer University of North Dakota

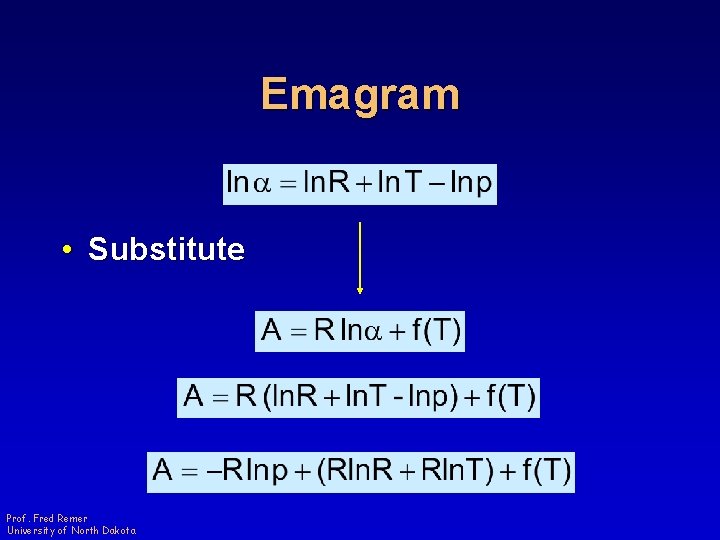
Emagram • Substitute Prof. Fred Remer University of North Dakota

Emagram • Select f(t) such that • Finally … coordinates A & B are … Prof. Fred Remer University of North Dakota

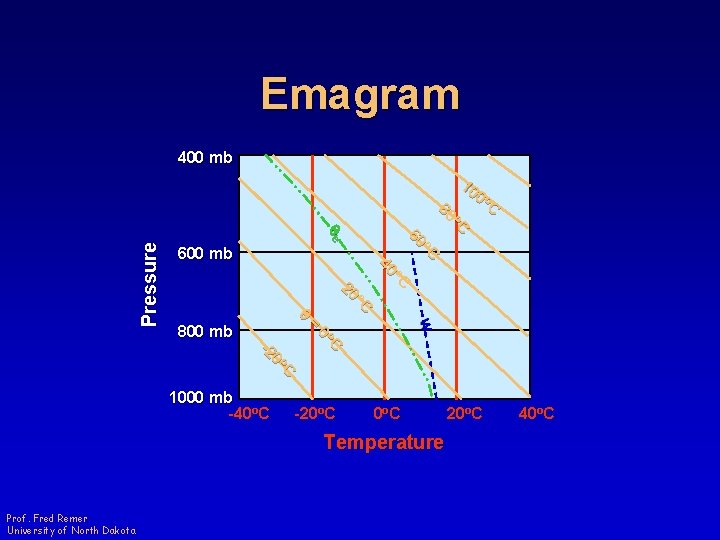
Emagram 400 mb o. C w = 800 mb o. C 40 o. C 20 q Pressure o. C 80 60 qe 600 mb 10 0 o C o. C 0 -2 1000 mb -40 o. C -20 o. C 0 o C Temperature Prof. Fred Remer University of North Dakota 20 o. C 40 o. C

Emagram • Area proportional to energy • Four sets of straight (or nearly straight) lines • 45 o angle between adiabats and isotherms Prof. Fred Remer University of North Dakota

Tephigram • T- f Diagram – Temperature = T – Entropy = f Prof. Fred Remer University of North Dakota

Tephigram • Coordinates – Similar to Emagram – Different constant of integration Prof. Fred Remer University of North Dakota

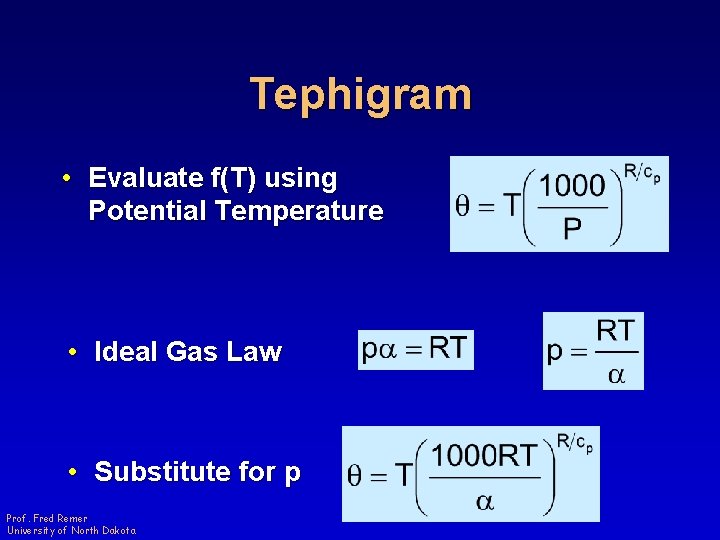
Tephigram • Evaluate f(T) using Potential Temperature • Ideal Gas Law • Substitute for p Prof. Fred Remer University of North Dakota

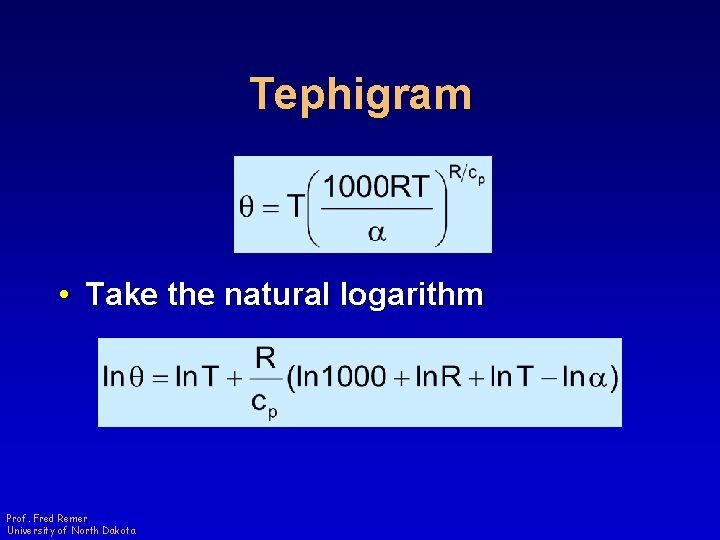
Tephigram • Take the natural logarithm Prof. Fred Remer University of North Dakota

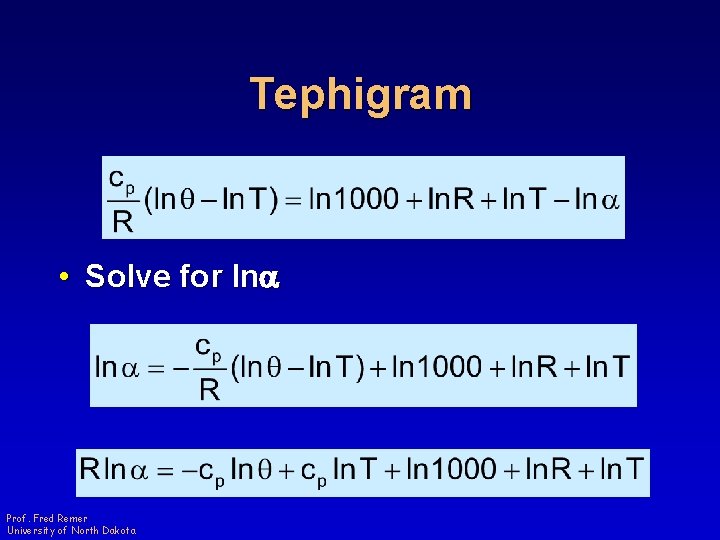
Tephigram • Solve for lna Prof. Fred Remer University of North Dakota

Tephigram • Solve for lna Prof. Fred Remer University of North Dakota

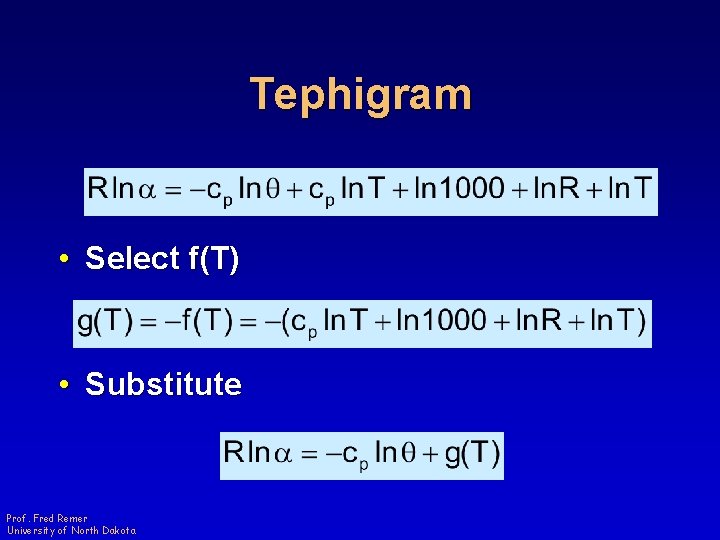
Tephigram • Select f(T) • Substitute Prof. Fred Remer University of North Dakota

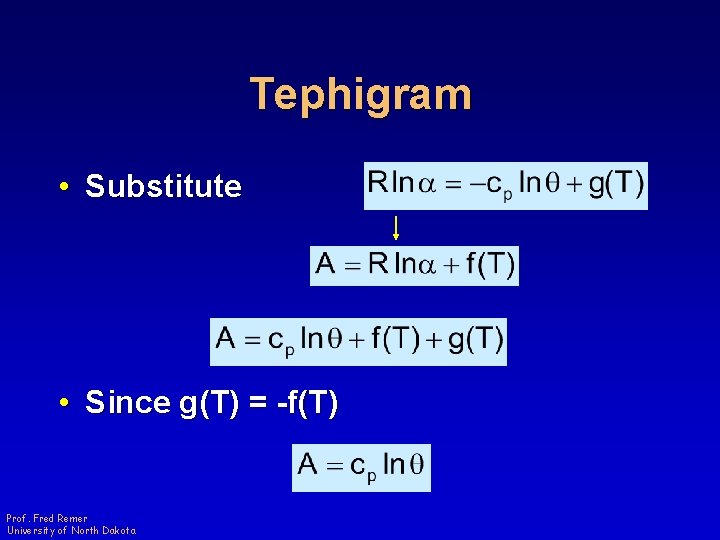
Tephigram • Substitute • Since g(T) = -f(T) Prof. Fred Remer University of North Dakota

Tephigram • Coordinates – Similar to Emagram Prof. Fred Remer University of North Dakota

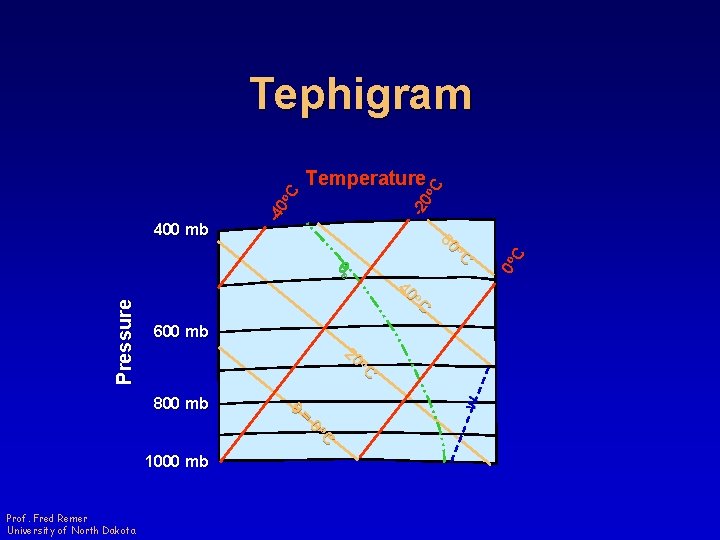
-2 0 o C Temperature o. C 60 o. C 40 q 800 mb w o. C 20 Pressure qe 600 mb = o. C 0 1000 mb Prof. Fred Remer University of North Dakota 0 o C 400 mb -4 0 o C Tephigram

Tephigram • Area proportional to energy • Four sets of straight (or nearly straight) lines – Isobars Curved! • 90 o angle between adiabats and isotherms Prof. Fred Remer University of North Dakota

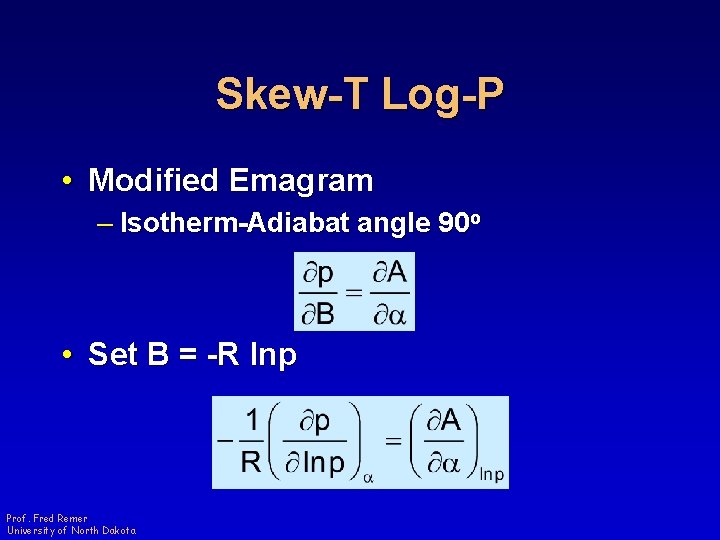
Skew-T Log-P • Modified Emagram – Isotherm-Adiabat angle 90 o • Set B = -R lnp Prof. Fred Remer University of North Dakota

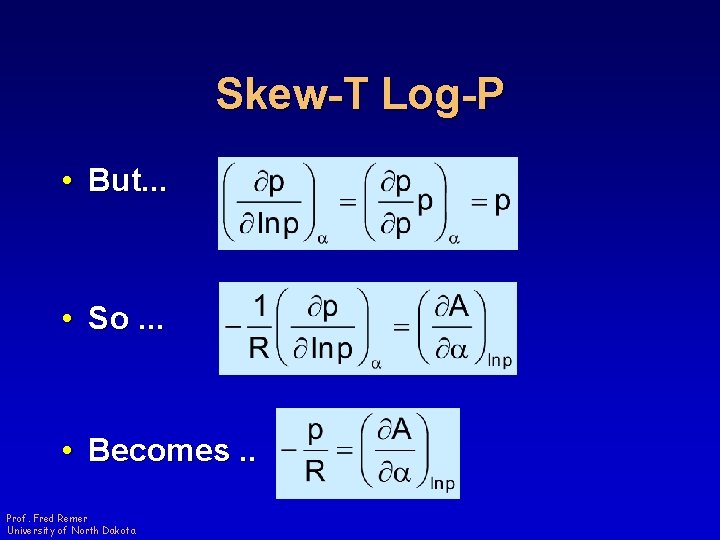
Skew-T Log-P • But. . . • So. . . • Becomes. . Prof. Fred Remer University of North Dakota

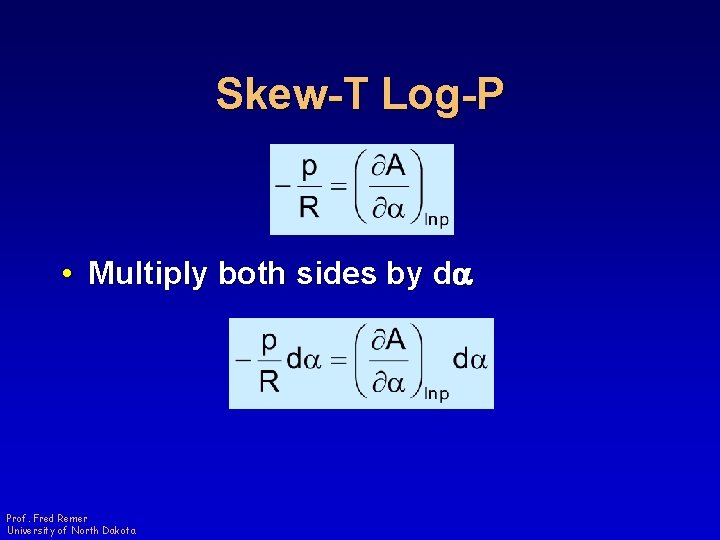
Skew-T Log-P • Multiply both sides by da Prof. Fred Remer University of North Dakota

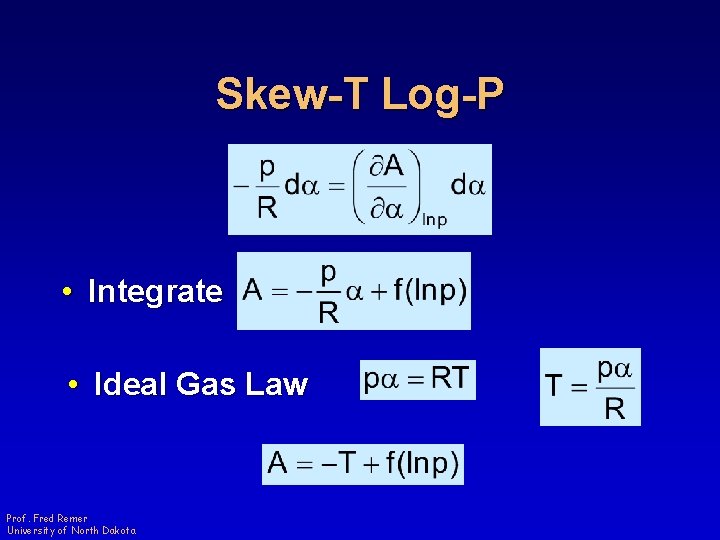
Skew-T Log-P • Integrate • Ideal Gas Law Prof. Fred Remer University of North Dakota

Skew-T Log-P • Select f(lnp) K = arbitrary constant Prof. Fred Remer University of North Dakota

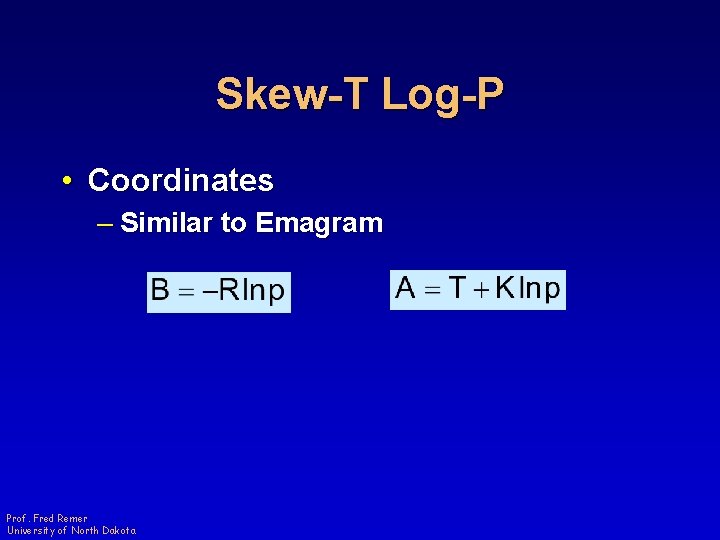
Skew-T Log-P • Coordinates – Similar to Emagram Prof. Fred Remer University of North Dakota

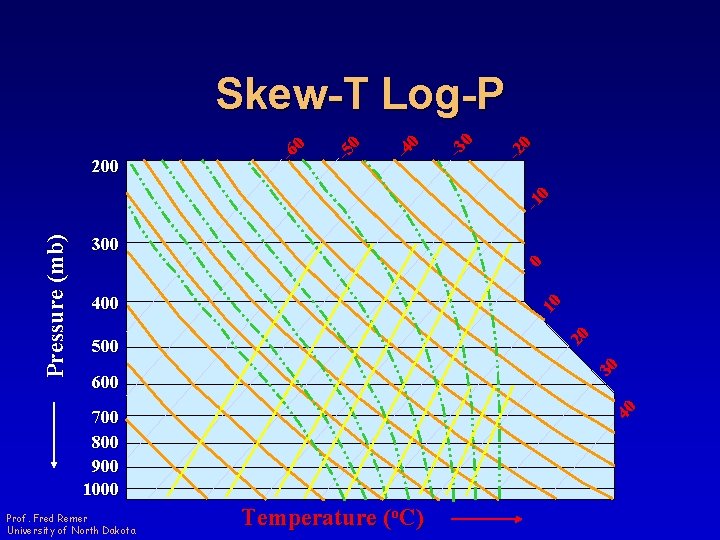
300 0 400 20 10 500 30 600 40 Pressure (mb) -1 0 -2 0 -3 0 -4 0 -5 0 200 -6 0 Skew-T Log-P 700 800 900 1000 Prof. Fred Remer University of North Dakota Temperature (o. C)

Skew-T Log-P • Area proportional to energy • Three set of straight lines – One set of gently curved lines – One set of curved lines • Adiabat-isotherm angle about 90 o Prof. Fred Remer University of North Dakota

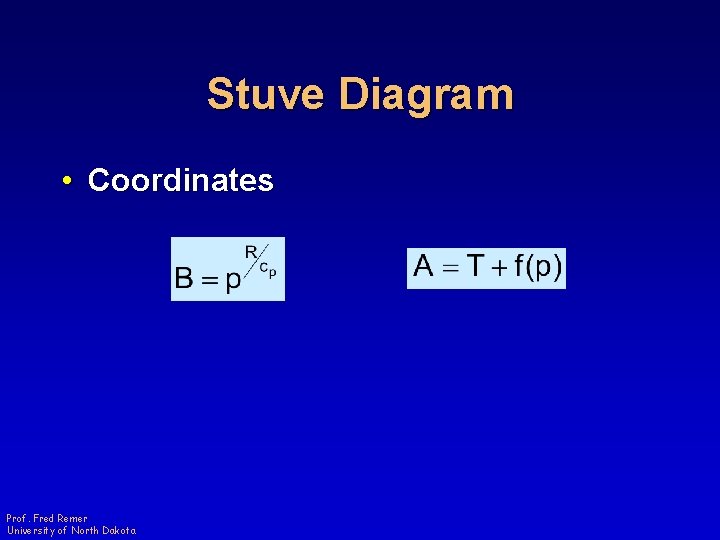
Stuve Diagram • Coordinates Prof. Fred Remer University of North Dakota

Stuve Diagram 400 Pressure 500 600 700 800 900 -40 o. C Prof. Fred Remer University of North Dakota -20 o. C Temperature 40 o. C

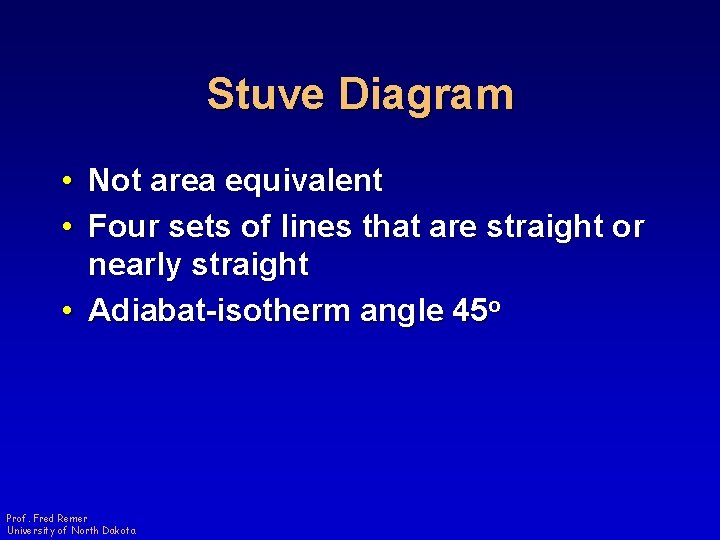
Stuve Diagram • Not area equivalent • Four sets of lines that are straight or nearly straight • Adiabat-isotherm angle 45 o Prof. Fred Remer University of North Dakota

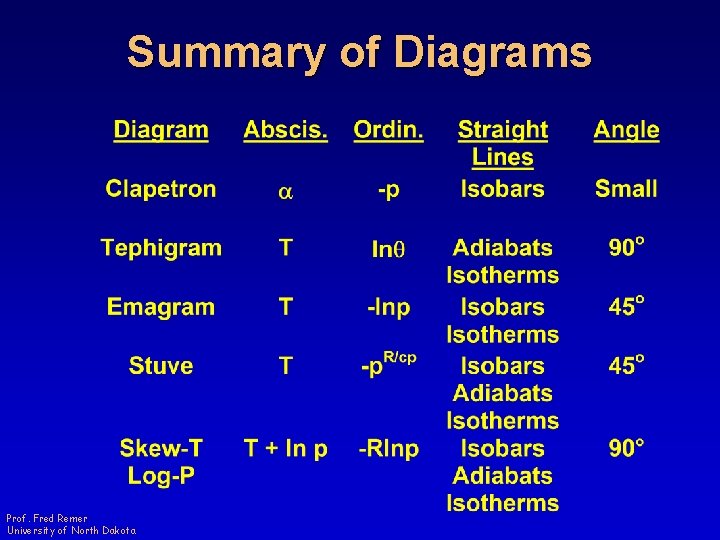
Summary of Diagrams Prof. Fred Remer University of North Dakota
 200 300 400
200 300 400 100 200 300 400 500 600 700 800 900
100 200 300 400 500 600 700 800 900 200+400+600+800
200+400+600+800 400 + 300 + 300
400 + 300 + 300 300+300+400
300+300+400 300 300 400
300 300 400 300+300+400
300+300+400 300+300+400
300+300+400 200+200+300
200+200+300 300+300+400
300+300+400 300+300+400
300+300+400 600+400+500
600+400+500 100 200 300 400 500
100 200 300 400 500 Signos romanos
Signos romanos 600+400
600+400 Sebuah bola kaki bermassa 500 gram
Sebuah bola kaki bermassa 500 gram 800+600+400
800+600+400 Nilai dari sin 600 + tg 600 adalah
Nilai dari sin 600 + tg 600 adalah 100 200 300 400
100 200 300 400 Who wrote this
Who wrote this 100 200 300 400
100 200 300 400 200 300 400
200 300 400 100+200+300+400
100+200+300+400 Normal distribution calculator math is fun
Normal distribution calculator math is fun Fry words 400-500
Fry words 400-500 200+200+300+300
200+200+300+300 200 300 300
200 300 300 300+300+200+200
300+300+200+200 100 + 100 + 200
100 + 100 + 200 Prime factorization of 200
Prime factorization of 200 Upper air charts
Upper air charts Upper air 850, 700, 500 & 300 mb charts
Upper air 850, 700, 500 & 300 mb charts 3500 / 500
3500 / 500 0700 400 0500
0700 400 0500 400 metros com barreiras
400 metros com barreiras Where is buddhism mostly located
Where is buddhism mostly located Diketahui luas selimut suatu tabung 880 cm
Diketahui luas selimut suatu tabung 880 cm Do you think m loisel enjoyed the ball
Do you think m loisel enjoyed the ball Jika besar sudut boc = 400 , maka besar sudut adc adalah
Jika besar sudut boc = 400 , maka besar sudut adc adalah Omni 400
Omni 400 Giasion 400
Giasion 400 Lkm 400 usm
Lkm 400 usm Advanced incident reporting system
Advanced incident reporting system 300-399 dewey decimal system
300-399 dewey decimal system Flagro fvo 400 troubleshooting
Flagro fvo 400 troubleshooting 7% z 400
7% z 400 400-800 nm
400-800 nm Saresp 2009 uma maquina fotografica
Saresp 2009 uma maquina fotografica Unlicensed two-way radios
Unlicensed two-way radios Logam tipis ukuran 1 cm * 5 cm dipanaskan sampai 400k
Logam tipis ukuran 1 cm * 5 cm dipanaskan sampai 400k Efavirenz 400 mg vs 600mg
Efavirenz 400 mg vs 600mg Kr-400
Kr-400 Mysteriously collapsed in 400 bc
Mysteriously collapsed in 400 bc Gcf of 12 and 24
Gcf of 12 and 24 3/4 of 400
3/4 of 400 Gabapentin 600 mg tablet picture
Gabapentin 600 mg tablet picture Technical poster
Technical poster