30 Orbitals and Organic Chemistry Pericyclic Reactions Based

30. Orbitals and Organic Chemistry: Pericyclic Reactions Based on Mc. Murry’s Organic Chemistry, 6 th edition © 2003 Ronald Kluger Department of Chemistry University of Toronto Based on Mc. Murry, Organic Chemistry, Chapter 30, 6 th edition, (c) 2003
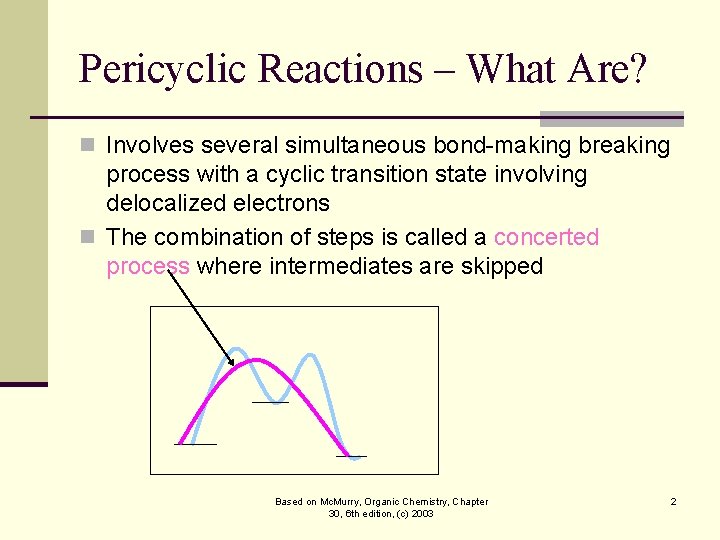
Pericyclic Reactions – What Are? n Involves several simultaneous bond-making breaking process with a cyclic transition state involving delocalized electrons n The combination of steps is called a concerted process where intermediates are skipped Based on Mc. Murry, Organic Chemistry, Chapter 30, 6 th edition, (c) 2003 2
30. 1 Molecular Orbitals of Conjugated Systems n A conjugated diene or polyene has alternating double and single bonds n Bonding MOs are lower in energy than the isolated p atomic orbitals and have the fewest nodes n Antibonding MOs are higher in energy n See Figure 30. 1 for a diagram Based on Mc. Murry, Organic Chemistry, Chapter 30, 6 th edition, (c) 2003 3

1, 3, 5 -Hexatriene n Three double bonds and six MOs n Only bonding orbitals, 1, 2, and 3, are filled in the ground state n On irradiation with ultraviolet light an electron is promoted from 3 to the lowest-energy unfilled orbital ( 4*) n This is the first (lowest energy) excited state n See the diagram in Figure 30. 2 Based on Mc. Murry, Organic Chemistry, Chapter 30, 6 th edition, (c) 2003 4
30. 2 Molecular Orbitals and Pericyclic Reactions n If the symmetries of both reactant and product orbitals match the reaction is said to be symmetry allowed under the Woodward-Hoffmann Rules (these relate the electronic configuration of reactants to the type of pericyclic reaction and its stereochemical imperatives) n If the symmetries of reactant and product orbitals do not correlate, the reaction is symmetry-disallowed and there no low energy concerted paths n Fukui’s approach: we need to consider only the highest occupied molecular orbital (HOMO) and the lowest unoccupied molecular orbital (LUMO), called the frontier orbitals Based on Mc. Murry, Organic Chemistry, Chapter 30, 6 th edition, (c) 2003 5
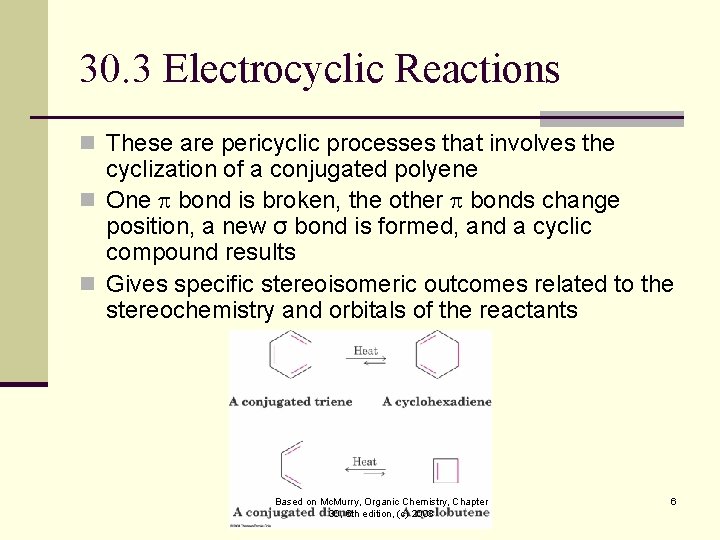
30. 3 Electrocyclic Reactions n These are pericyclic processes that involves the cyclization of a conjugated polyene n One bond is broken, the other bonds change position, a new σ bond is formed, and a cyclic compound results n Gives specific stereoisomeric outcomes related to the stereochemistry and orbitals of the reactants Based on Mc. Murry, Organic Chemistry, Chapter 30, 6 th edition, (c) 2003 6
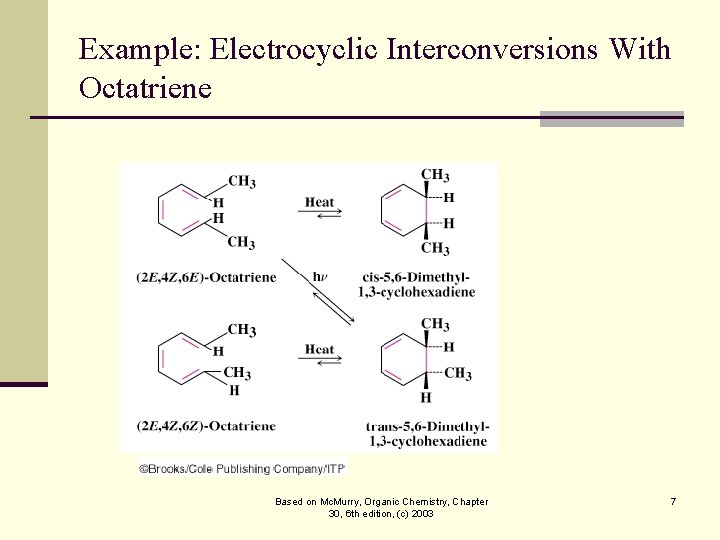
Example: Electrocyclic Interconversions With Octatriene Based on Mc. Murry, Organic Chemistry, Chapter 30, 6 th edition, (c) 2003 7
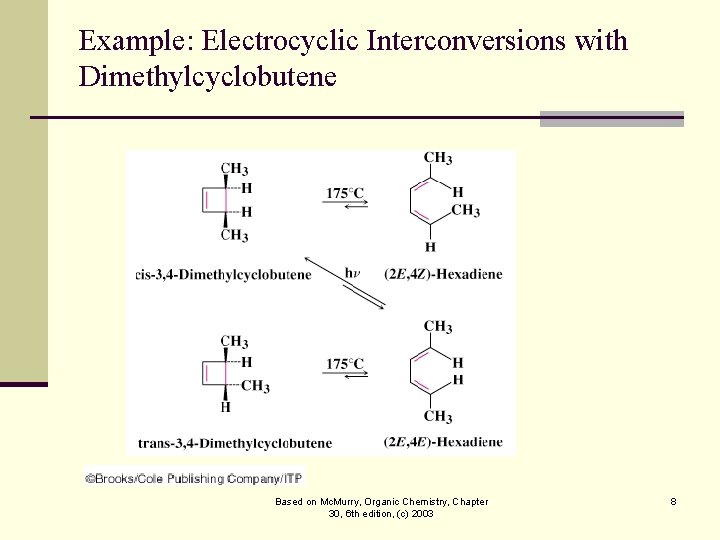
Example: Electrocyclic Interconversions with Dimethylcyclobutene Based on Mc. Murry, Organic Chemistry, Chapter 30, 6 th edition, (c) 2003 8
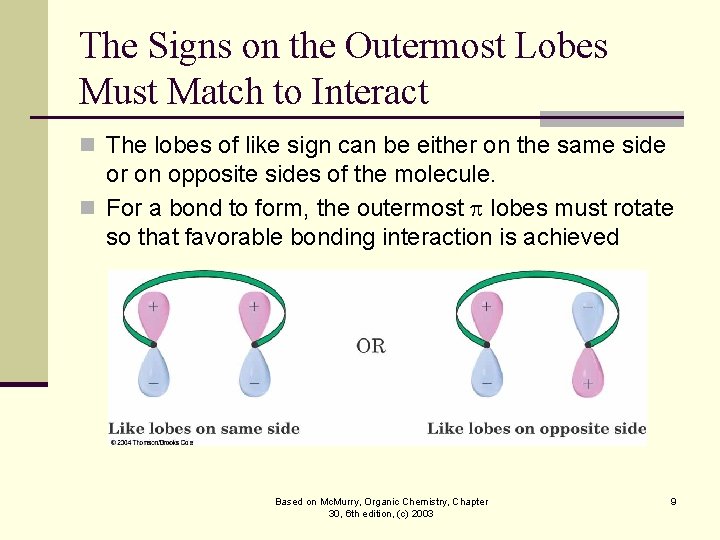
The Signs on the Outermost Lobes Must Match to Interact n The lobes of like sign can be either on the same side or on opposite sides of the molecule. n For a bond to form, the outermost lobes must rotate so that favorable bonding interaction is achieved Based on Mc. Murry, Organic Chemistry, Chapter 30, 6 th edition, (c) 2003 9
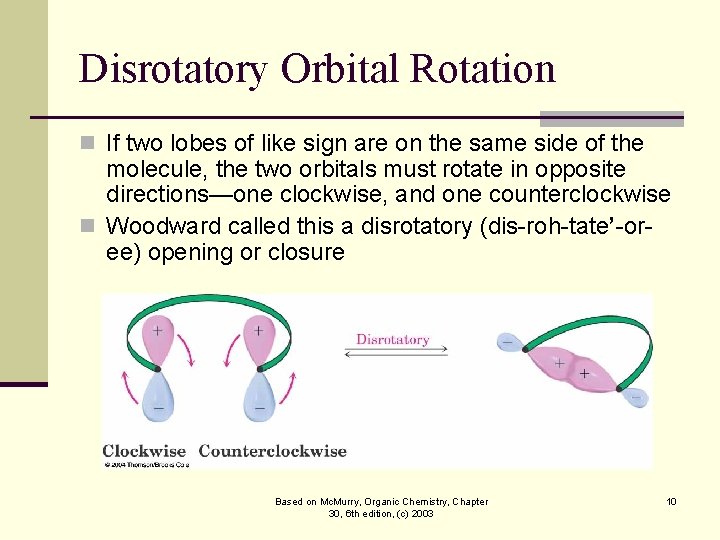
Disrotatory Orbital Rotation n If two lobes of like sign are on the same side of the molecule, the two orbitals must rotate in opposite directions—one clockwise, and one counterclockwise n Woodward called this a disrotatory (dis-roh-tate’-oree) opening or closure Based on Mc. Murry, Organic Chemistry, Chapter 30, 6 th edition, (c) 2003 10
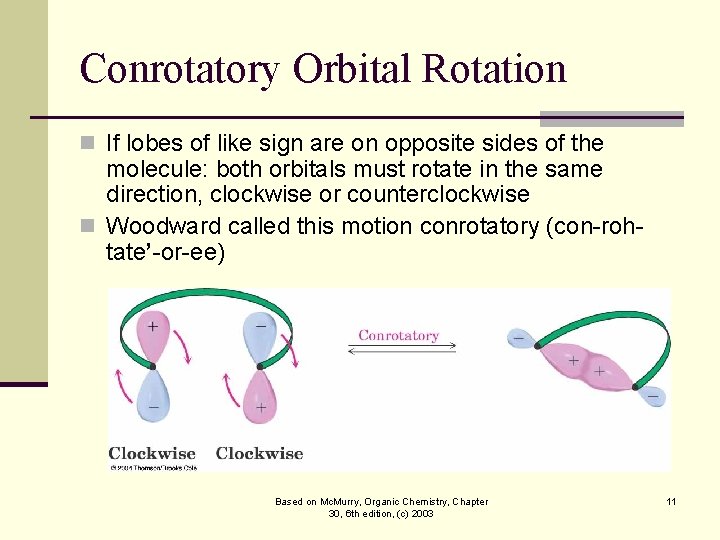
Conrotatory Orbital Rotation n If lobes of like sign are on opposite sides of the molecule: both orbitals must rotate in the same direction, clockwise or counterclockwise n Woodward called this motion conrotatory (con-rohtate’-or-ee) Based on Mc. Murry, Organic Chemistry, Chapter 30, 6 th edition, (c) 2003 11
30. 4 Stereochemistry of Thermal Electrocyclic Reactions n Determined by the symmetry of the polyene HOMO n The ground-state electronic configuration is used to identify the HOMO n (Photochemical reactions go through the excited-state electronic configuration ) Based on Mc. Murry, Organic Chemistry, Chapter 30, 6 th edition, (c) 2003 12
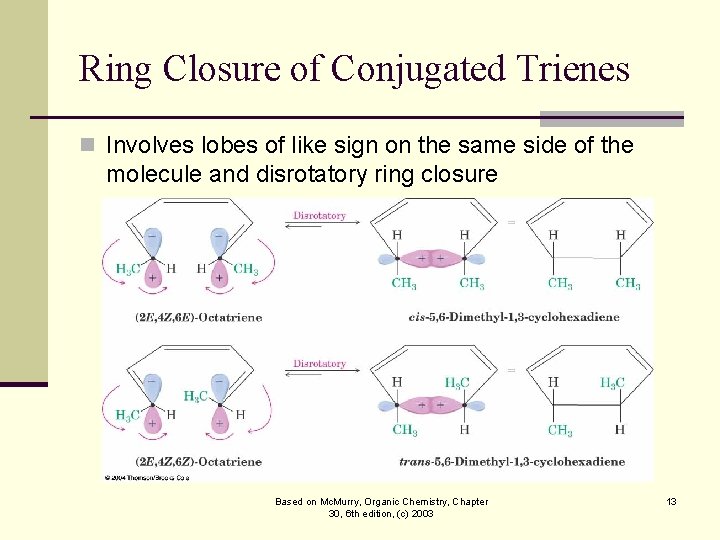
Ring Closure of Conjugated Trienes n Involves lobes of like sign on the same side of the molecule and disrotatory ring closure Based on Mc. Murry, Organic Chemistry, Chapter 30, 6 th edition, (c) 2003 13
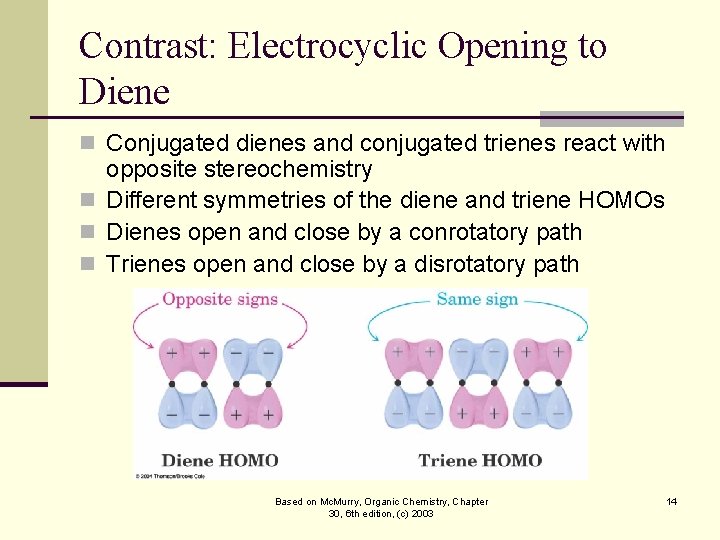
Contrast: Electrocyclic Opening to Diene n Conjugated dienes and conjugated trienes react with opposite stereochemistry n Different symmetries of the diene and triene HOMOs n Dienes open and close by a conrotatory path n Trienes open and close by a disrotatory path Based on Mc. Murry, Organic Chemistry, Chapter 30, 6 th edition, (c) 2003 14
30. 5 Photochemical Electrocyclic Reactions n Irradiation of a polyene excites one electron from HOMO to LUMO n This causes the old LUMO to become the new HOMO, with changed symmetry n This changes the reaction stereochemistry (symmetries of thermal and photochemical electrocylic reactions are always opposite) Based on Mc. Murry, Organic Chemistry, Chapter 30, 6 th edition, (c) 2003 15
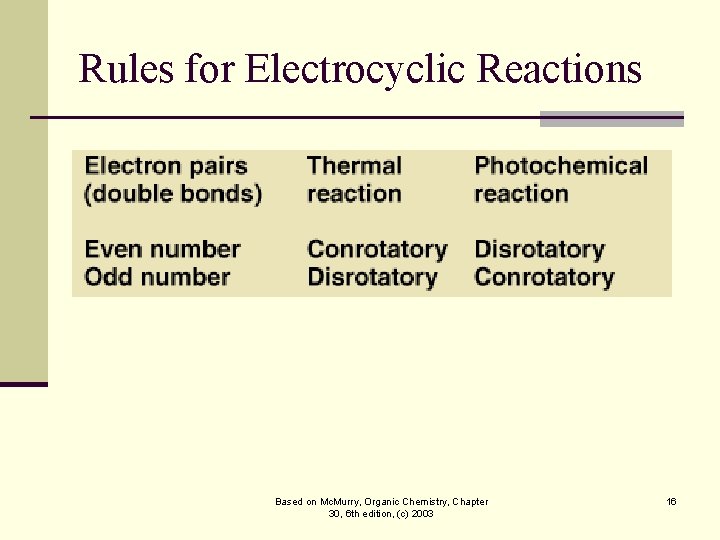
Rules for Electrocyclic Reactions Based on Mc. Murry, Organic Chemistry, Chapter 30, 6 th edition, (c) 2003 16
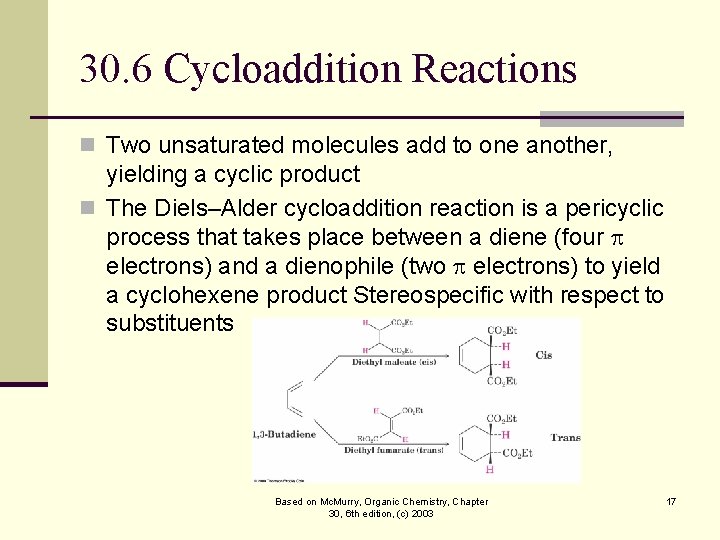
30. 6 Cycloaddition Reactions n Two unsaturated molecules add to one another, yielding a cyclic product n The Diels–Alder cycloaddition reaction is a pericyclic process that takes place between a diene (four electrons) and a dienophile (two electrons) to yield a cyclohexene product Stereospecific with respect to substituents Based on Mc. Murry, Organic Chemistry, Chapter 30, 6 th edition, (c) 2003 17
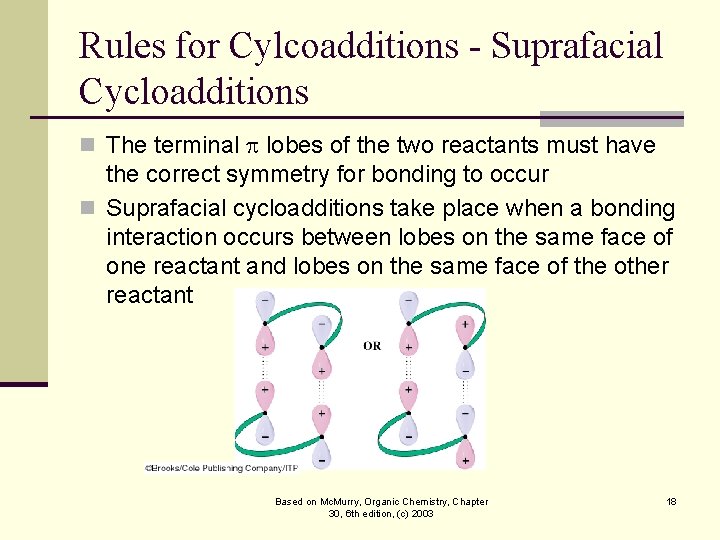
Rules for Cylcoadditions - Suprafacial Cycloadditions n The terminal lobes of the two reactants must have the correct symmetry for bonding to occur n Suprafacial cycloadditions take place when a bonding interaction occurs between lobes on the same face of one reactant and lobes on the same face of the other reactant Based on Mc. Murry, Organic Chemistry, Chapter 30, 6 th edition, (c) 2003 18
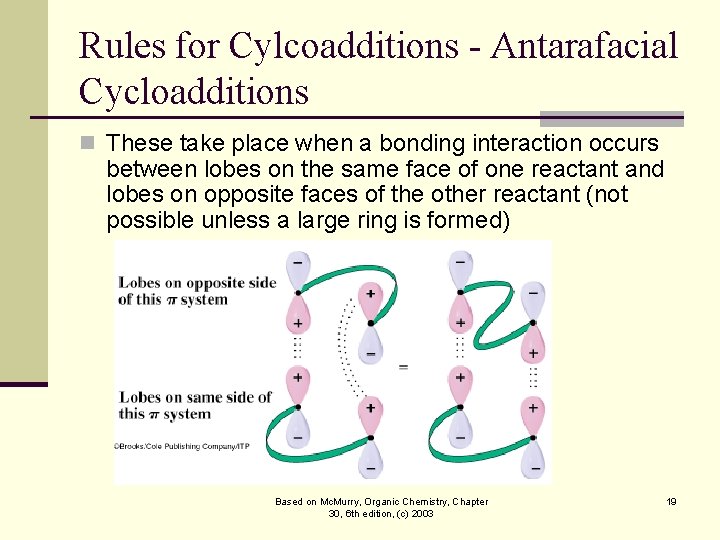
Rules for Cylcoadditions - Antarafacial Cycloadditions n These take place when a bonding interaction occurs between lobes on the same face of one reactant and lobes on opposite faces of the other reactant (not possible unless a large ring is formed) Based on Mc. Murry, Organic Chemistry, Chapter 30, 6 th edition, (c) 2003 19
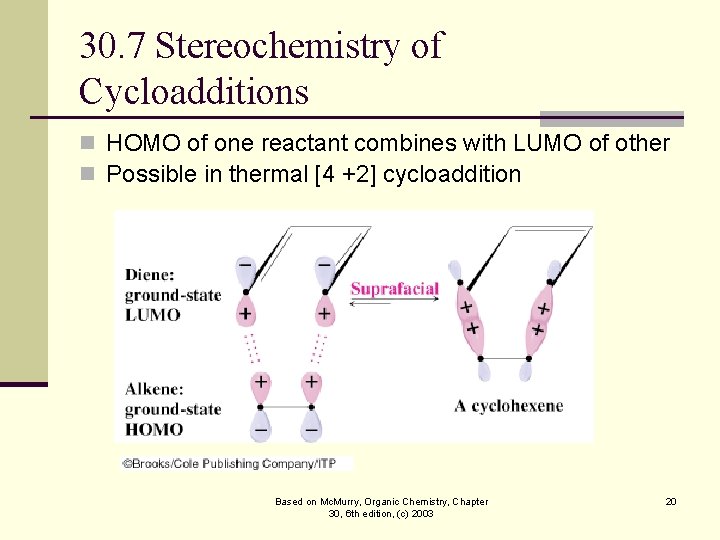
30. 7 Stereochemistry of Cycloadditions n HOMO of one reactant combines with LUMO of other n Possible in thermal [4 +2] cycloaddition Based on Mc. Murry, Organic Chemistry, Chapter 30, 6 th edition, (c) 2003 20
![[2+2] Cylcoadditions n Only the excited-state HOMO of one alkene and the LUMO can [2+2] Cylcoadditions n Only the excited-state HOMO of one alkene and the LUMO can](http://slidetodoc.com/presentation_image_h2/71ddb43d97d49ced7e783bf79f0d5263/image-21.jpg)
[2+2] Cylcoadditions n Only the excited-state HOMO of one alkene and the LUMO can combine by a suprafacial pathway in the combination of two alkenes Based on Mc. Murry, Organic Chemistry, Chapter 30, 6 th edition, (c) 2003 21
![Formation of Four-Membered Rings n Photochemical [2 + 2] cycloaddition reaction occurs smoothly Based Formation of Four-Membered Rings n Photochemical [2 + 2] cycloaddition reaction occurs smoothly Based](http://slidetodoc.com/presentation_image_h2/71ddb43d97d49ced7e783bf79f0d5263/image-22.jpg)
Formation of Four-Membered Rings n Photochemical [2 + 2] cycloaddition reaction occurs smoothly Based on Mc. Murry, Organic Chemistry, Chapter 30, 6 th edition, (c) 2003 22
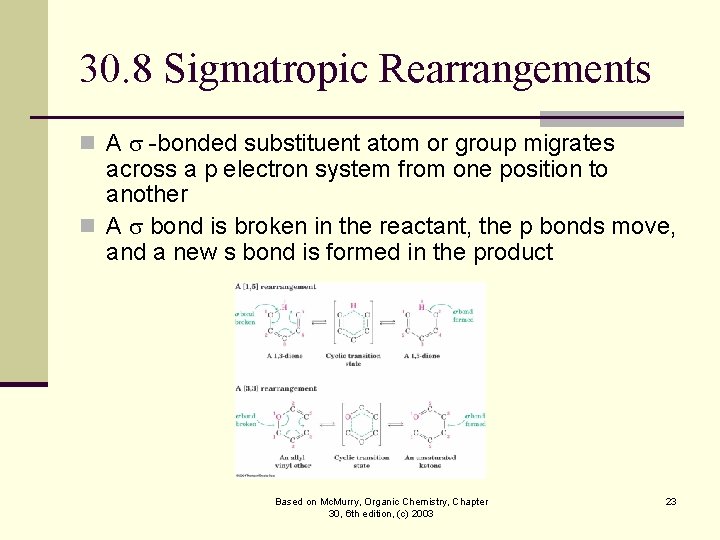
30. 8 Sigmatropic Rearrangements n A s -bonded substituent atom or group migrates across a p electron system from one position to another n A s bond is broken in the reactant, the p bonds move, and a new s bond is formed in the product Based on Mc. Murry, Organic Chemistry, Chapter 30, 6 th edition, (c) 2003 23
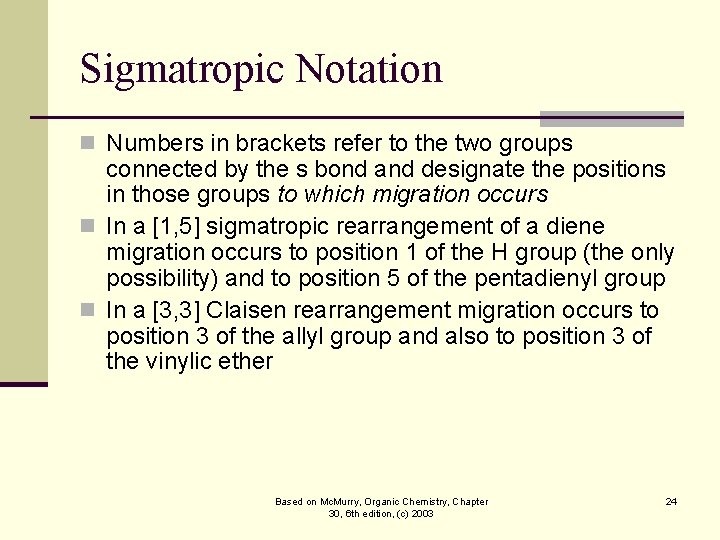
Sigmatropic Notation n Numbers in brackets refer to the two groups connected by the s bond and designate the positions in those groups to which migration occurs n In a [1, 5] sigmatropic rearrangement of a diene migration occurs to position 1 of the H group (the only possibility) and to position 5 of the pentadienyl group n In a [3, 3] Claisen rearrangement migration occurs to position 3 of the allyl group and also to position 3 of the vinylic ether Based on Mc. Murry, Organic Chemistry, Chapter 30, 6 th edition, (c) 2003 24
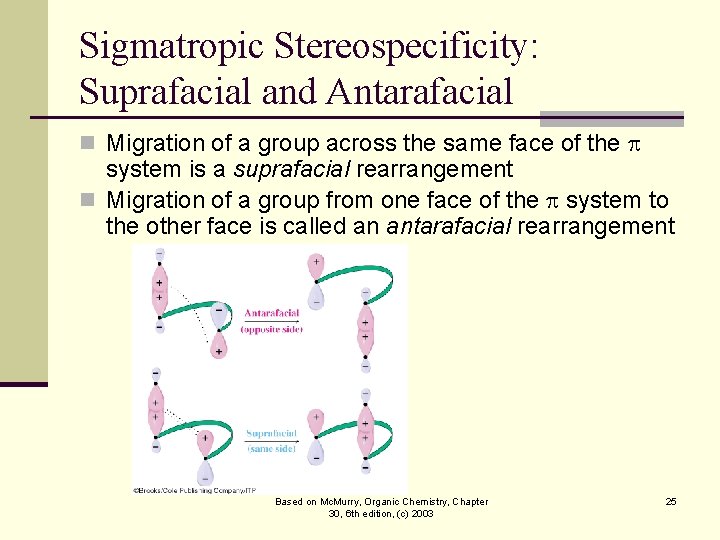
Sigmatropic Stereospecificity: Suprafacial and Antarafacial n Migration of a group across the same face of the system is a suprafacial rearrangement n Migration of a group from one face of the system to the other face is called an antarafacial rearrangement Based on Mc. Murry, Organic Chemistry, Chapter 30, 6 th edition, (c) 2003 25
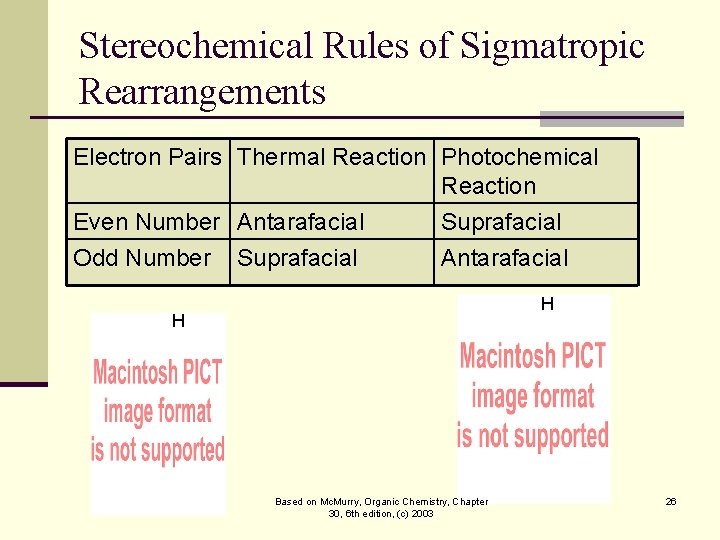
Stereochemical Rules of Sigmatropic Rearrangements Electron Pairs Thermal Reaction Photochemical Reaction Even Number Antarafacial Odd Number Suprafacial Antarafacial H H Based on Mc. Murry, Organic Chemistry, Chapter 30, 6 th edition, (c) 2003 26
![30. 9 Some Examples of Sigmatropic Rearrangements n A [1, 5] sigmatropic rearrangement involves 30. 9 Some Examples of Sigmatropic Rearrangements n A [1, 5] sigmatropic rearrangement involves](http://slidetodoc.com/presentation_image_h2/71ddb43d97d49ced7e783bf79f0d5263/image-27.jpg)
30. 9 Some Examples of Sigmatropic Rearrangements n A [1, 5] sigmatropic rearrangement involves three electron pairs (two bonds and one s bond) n Orbital-symmetry rules predict a suprafacial reaction n 5 -methylcyclopentadiene rapidly rearranges at room temperature Based on Mc. Murry, Organic Chemistry, Chapter 30, 6 th edition, (c) 2003 27
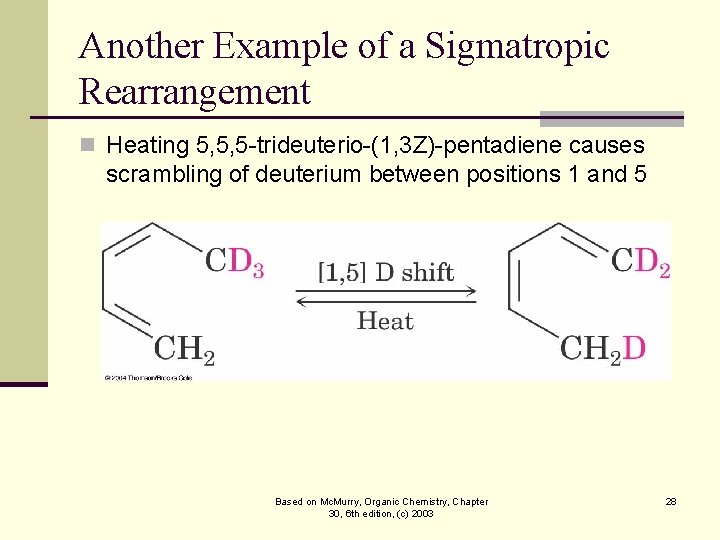
Another Example of a Sigmatropic Rearrangement n Heating 5, 5, 5 -trideuterio-(1, 3 Z)-pentadiene causes scrambling of deuterium between positions 1 and 5 Based on Mc. Murry, Organic Chemistry, Chapter 30, 6 th edition, (c) 2003 28
![Orbital Picture of a Suprafacial [1, 5] H Shift Based on Mc. Murry, Organic Orbital Picture of a Suprafacial [1, 5] H Shift Based on Mc. Murry, Organic](http://slidetodoc.com/presentation_image_h2/71ddb43d97d49ced7e783bf79f0d5263/image-29.jpg)
Orbital Picture of a Suprafacial [1, 5] H Shift Based on Mc. Murry, Organic Chemistry, Chapter 30, 6 th edition, (c) 2003 29
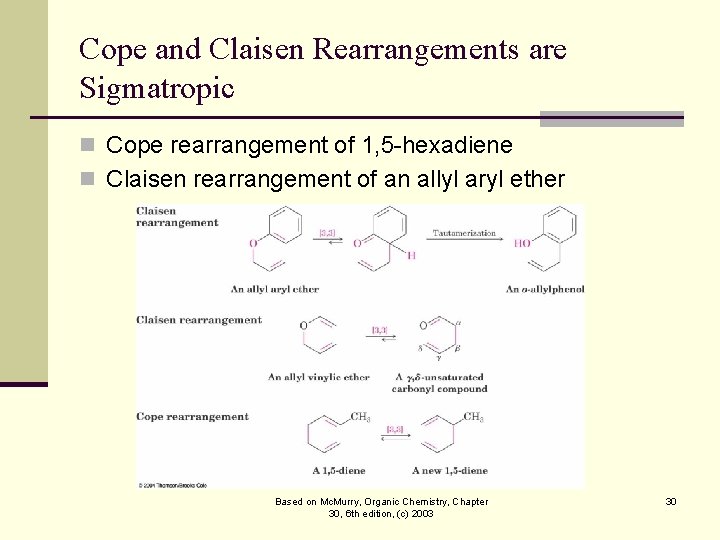
Cope and Claisen Rearrangements are Sigmatropic n Cope rearrangement of 1, 5 -hexadiene n Claisen rearrangement of an allyl aryl ether Based on Mc. Murry, Organic Chemistry, Chapter 30, 6 th edition, (c) 2003 30
![Suprafacial [3, 3] Cope and Claisen Rearrangements n Both involve reorganization of an odd Suprafacial [3, 3] Cope and Claisen Rearrangements n Both involve reorganization of an odd](http://slidetodoc.com/presentation_image_h2/71ddb43d97d49ced7e783bf79f0d5263/image-31.jpg)
Suprafacial [3, 3] Cope and Claisen Rearrangements n Both involve reorganization of an odd number of electron pairs (two bonds and one s bond) n Both react by suprafacial pathways Based on Mc. Murry, Organic Chemistry, Chapter 30, 6 th edition, (c) 2003 31
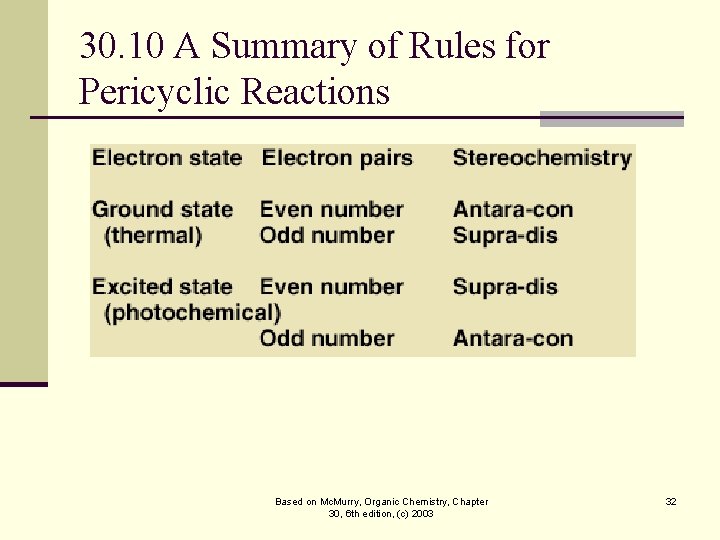
30. 10 A Summary of Rules for Pericyclic Reactions Based on Mc. Murry, Organic Chemistry, Chapter 30, 6 th edition, (c) 2003 32
- Slides: 32