30 day Outcomes of Transcatheter Mitral Valve Replacement





- Slides: 15

30 -day Outcomes of Transcatheter Mitral Valve Replacement in Native Mitral Valve Disease with Severe Mitral Annular Calcification in the United States: Data from the STS/ACC/TVT Registry Mayra Guerrero, MD, FACC, FSCAI Evanston Hospital On behalf of the TVT Registry Euro. PCR 2018 Paris, France May 23 th, 2018 Evanston Hospital

Funding Support and Disclaimer This research was supported by the American College of Cardiology Foundation’s National Cardiovascular Data Registry (NCDR) and The Society of Thoracic Surgeons National Database. The views expressed in this presentation represent those of the author(s), and do not necessarily represent the official views of either organization. Learn more about the STS/ACC TVT Registry at www. tvtregistry. org.

Disclosure Statement of Financial Interest Within the past 12 months, I or my spouse/partner have had a financial interest/arrangement or affiliation with the organization(s) listed below. Affiliation/Financial Relationship • Research Grant Support, Proctor • Consultant, Speaker’s Bureau • Research Grant Company • Edwards Lifesciences • Abbott, Boston Scientific • ACC/STS NCDR Off label use of products and investigational devices will be discussed in this presentation Evanston Hospital

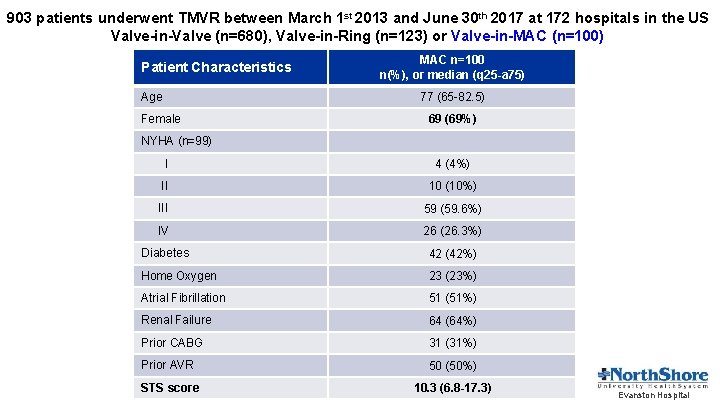
903 patients underwent TMVR between March 1 st 2013 and June 30 th 2017 at 172 hospitals in the US Valve-in-Valve (n=680), Valve-in-Ring (n=123) or Valve-in-MAC (n=100) Patient Characteristics Age MAC n=100 n(%), or median (q 25 -a 75) 77 (65 -82. 5) Female 69 (69%) NYHA (n=99) I 4 (4%) II 10 (10%) III 59 (59. 6%) IV 26 (26. 3%) Diabetes 42 (42%) Home Oxygen 23 (23%) Atrial Fibrillation 51 (51%) Renal Failure 64 (64%) Prior CABG 31 (31%) Prior AVR 50 (50%) STS score 10. 3 (6. 8 -17. 3) Evanston Hospital

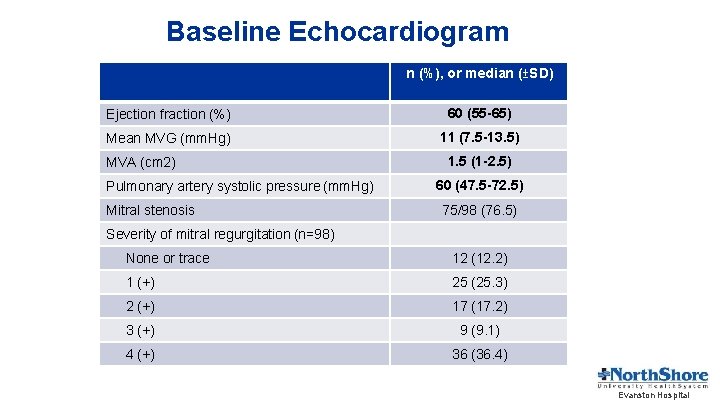
Baseline Echocardiogram n (%), or median (±SD) Ejection fraction (%) 60 (55 -65) Mean MVG (mm. Hg) 11 (7. 5 -13. 5) MVA (cm 2) Pulmonary artery systolic pressure (mm. Hg) Mitral stenosis 1. 5 (1 -2. 5) 60 (47. 5 -72. 5) 75/98 (76. 5) Severity of mitral regurgitation (n=98) None or trace 12 (12. 2) 1 (+) 25 (25. 3) 2 (+) 17 (17. 2) 3 (+) 9 (9. 1) 4 (+) 36 (36. 4) Evanston Hospital

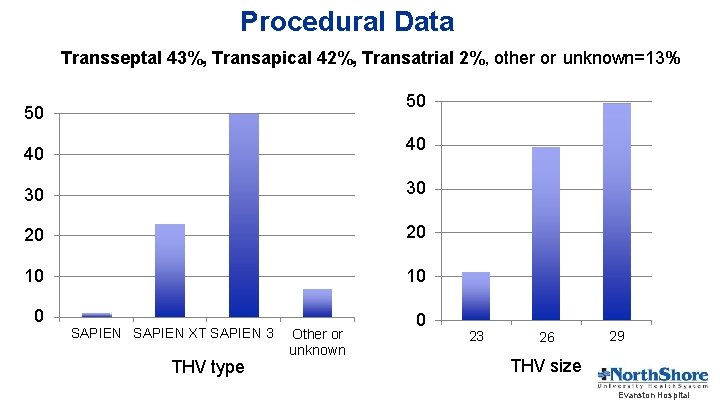
Procedural Data Transseptal 43%, Transapical 42%, Transatrial 2%, other or unknown=13% 50 50 40 40 30 30 20 20 10 10 0 SAPIEN XT SAPIEN 3 THV type Other or unknown 0 23 26 29 THV size Evanston Hospital

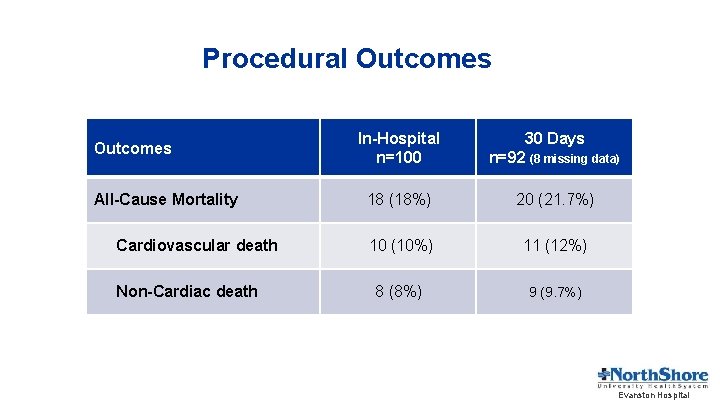
Procedural Outcomes All-Cause Mortality Cardiovascular death Non-Cardiac death In-Hospital n=100 30 Days n=92 (8 missing data) 18 (18%) 20 (21. 7%) 10 (10%) 11 (12%) 8 (8%) 9 (9. 7%) Evanston Hospital

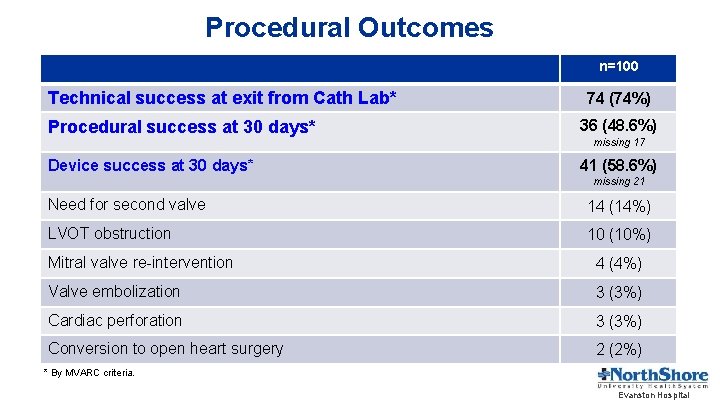
Procedural Outcomes n=100 Technical success at exit from Cath Lab* 74 (74%) Procedural success at 30 days* 36 (48. 6%) Device success at 30 days* 41 (58. 6%) missing 17 missing 21 Need for second valve 14 (14%) LVOT obstruction 10 (10%) Mitral valve re-intervention 4 (4%) Valve embolization 3 (3%) Cardiac perforation 3 (3%) Conversion to open heart surgery 2 (2%) * By MVARC criteria. Evanston Hospital

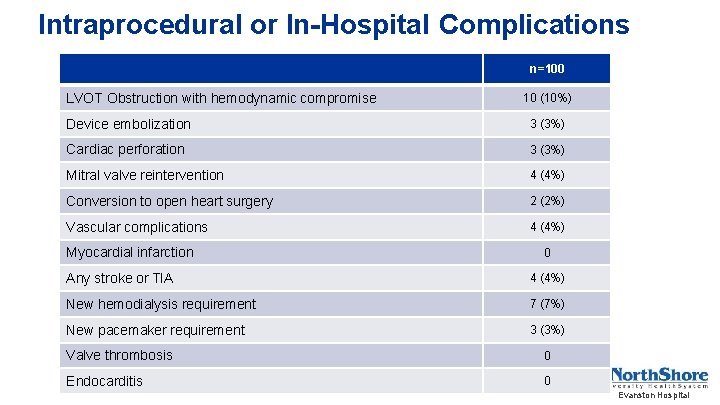
Intraprocedural or In-Hospital Complications n=100 LVOT Obstruction with hemodynamic compromise 10 (10%) Device embolization 3 (3%) Cardiac perforation 3 (3%) Mitral valve reintervention 4 (4%) Conversion to open heart surgery 2 (2%) Vascular complications 4 (4%) Myocardial infarction 0 Any stroke or TIA 4 (4%) New hemodialysis requirement 7 (7%) New pacemaker requirement 3 (3%) Valve thrombosis 0 Endocarditis 0 Evanston Hospital

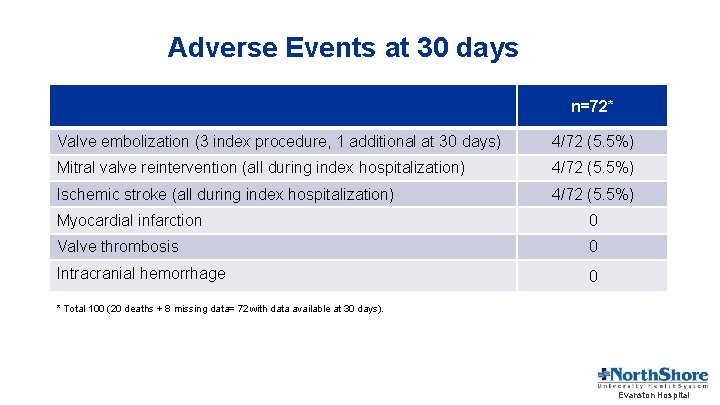
Adverse Events at 30 days n=72* Valve embolization (3 index procedure, 1 additional at 30 days) 4/72 (5. 5%) Mitral valve reintervention (all during index hospitalization) 4/72 (5. 5%) Ischemic stroke (all during index hospitalization) 4/72 (5. 5%) Myocardial infarction 0 Valve thrombosis 0 Intracranial hemorrhage 0 * Total 100 (20 deaths + 8 missing data= 72 with data available at 30 days). Evanston Hospital

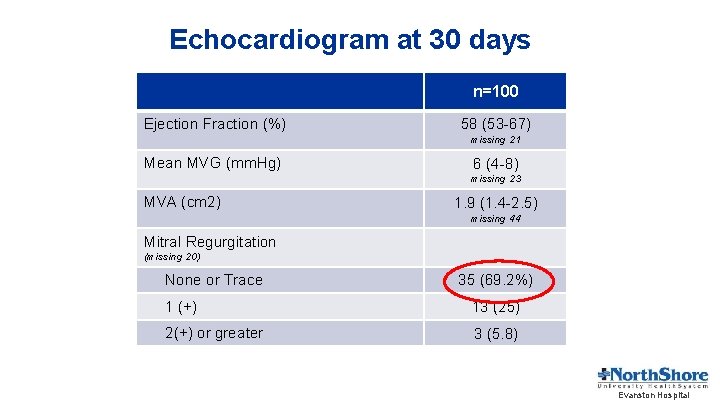
Echocardiogram at 30 days n=100 Ejection Fraction (%) 58 (53 -67) missing 21 Mean MVG (mm. Hg) 6 (4 -8) missing 23 MVA (cm 2) 1. 9 (1. 4 -2. 5) missing 44 Mitral Regurgitation (missing 20) None or Trace 35 (69. 2%) 1 (+) 13 (25) 2(+) or greater 3 (5. 8) Evanston Hospital

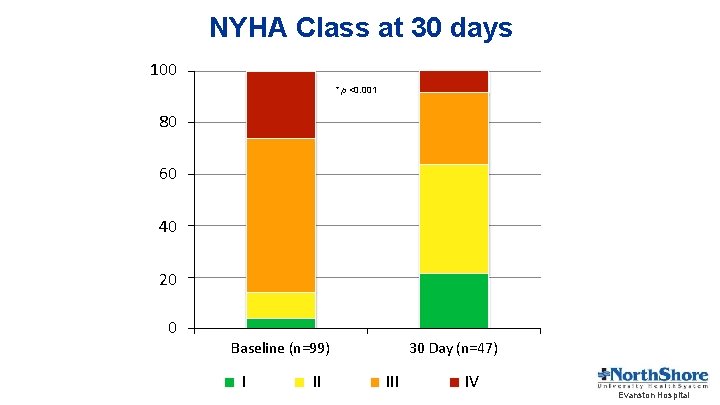
NYHA Class at 30 days 100 * p <0. 001 80 60 40 20 0 30 Day (n=47) Baseline (n=99) I II IV Evanston Hospital

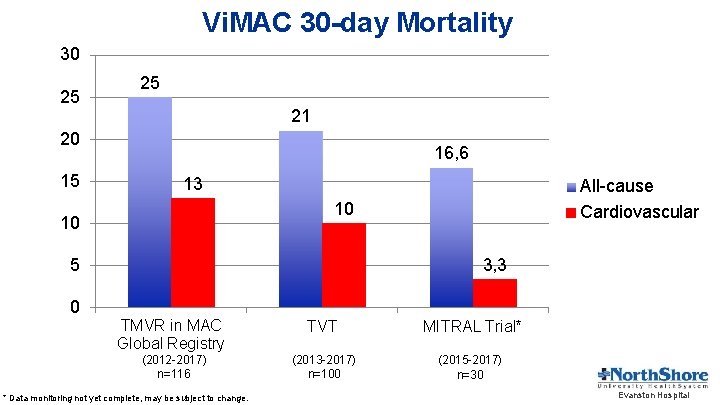
Vi. MAC 30 -day Mortality 30 25 25 21 20 15 16, 6 13 10 10 5 0 All-cause Cardiovascular 3, 3 TMVR in MAC Global Registry TVT MITRAL Trial* (2012 -2017) n=116 (2013 -2017) n=100 (2015 -2017) n=30 * Data monitoring not yet complete, may be subject to change. Evanston Hospital

Conclusions TMVR with balloon expandable aortic THVs is feasible in severe MAC It is a challenging procedure associated with complications and mortality Patients who survive the procedure, experience improvement of symptoms Valve function was maintained at 30 days in this early experience Outcomes are improving with better patient selection and techniques Further efforts are needed to improve overall outcomes The long term effect is not known and requires evaluation Evanston Hospital

Thank You mayraguerrero@me. com