3 Safety Considerations Safety Overview n Impart basic

3) Safety Considerations Safety Overview n Impart basic knowledge of pressure and the forces exerted. n Understand how temperature causes burn injury. n Examples of Safety Incidents n Understand Site Safety values and systems. n 1

Safety is a Value n No work should be started if it cannot be completed safely. n The goal is that personnel will go home as they arrived - safe and without injury. n Personnel should not be injured in the manufacture of a product, which is designed to improve the quality of life. 2
Hazardous Process Safety n Examples of Hazardous Processes Are: Vessels (Pressurized) n Plant steam (60 psi, 150 C) n Clean steam (20 -30 psi) n WFI (Water for Injection) n Compressed air (95 -105 psi) n CIP systems (~100 psi, ~80 C, CORROSIVE) n Solution transfer lines n Oxygen and nitrogen gas (& cylinders) n 3

Hazardous Process Safety n Possible Hazards: Impact from a blast or release of compressed liquid or gas n Traumatic injury from flying parts n n Burns from the release of hot liquid or gasses 4 n Contact with released liquid or gas (chemical burns) n Fire resulting from the escape of flammable liquid or gas (e. g. ethyl alcohol or oxygen

Safety Considerations Are you aware of the hazards of pressure and temperature? 5

Pressure Vessel Safety Considerations This is a processing vessel…. . but its also a timebomb, waiting to go off in your face. 6
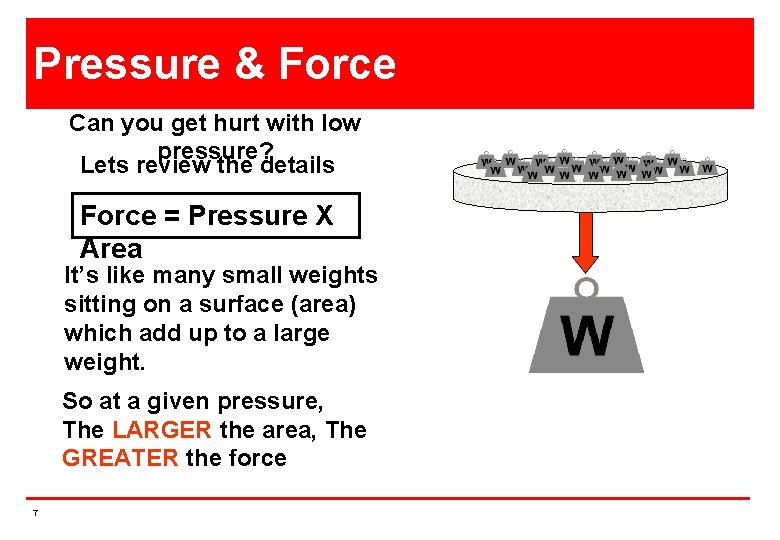
Pressure & Force Can you get hurt with low pressure? Lets review the details Force = Pressure X Area It’s like many small weights sitting on a surface (area) which add up to a large weight. So at a given pressure, The LARGER the area, The GREATER the force 7
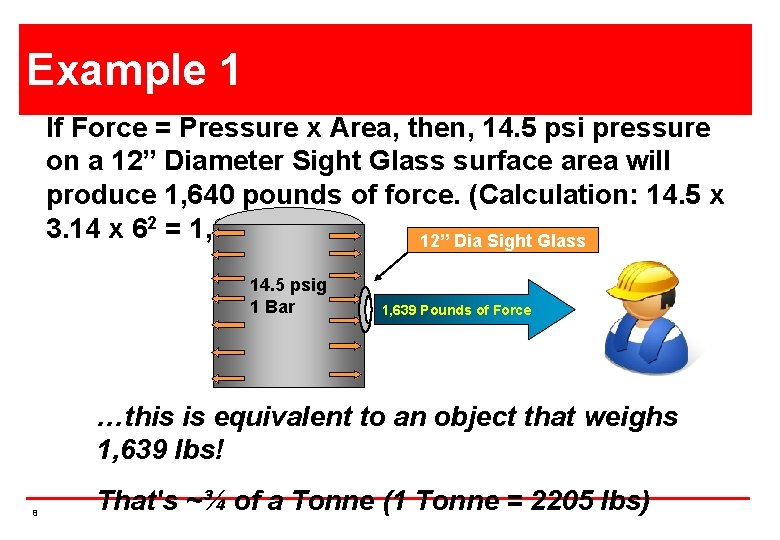
Example 1 If Force = Pressure x Area, then, 14. 5 psi pressure on a 12” Diameter Sight Glass surface area will produce 1, 640 pounds of force. (Calculation: 14. 5 x 3. 14 x 62 = 1, 639 lbs) 12”X Dia Glass 12” Sight Square 14. 5 psig 1 Bar 1, 639 Pounds of Force …this is equivalent to an object that weighs 1, 639 lbs! 8 That's ~¾ of a Tonne (1 Tonne = 2205 lbs)
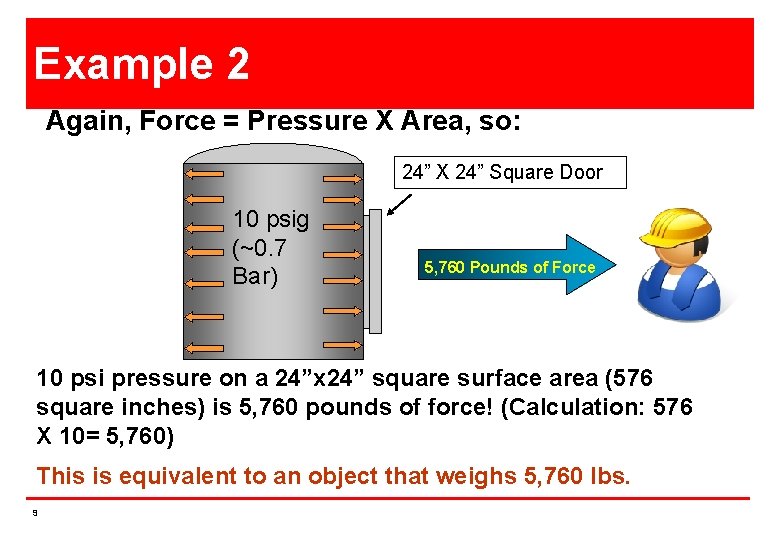
Example 2 Again, Force = Pressure X Area, so: 24” X 24” Square Door 10 psig (~0. 7 Bar) 5, 760 Pounds of Force 10 psi pressure on a 24”x 24” square surface area (576 square inches) is 5, 760 pounds of force! (Calculation: 576 X 10= 5, 760) This is equivalent to an object that weighs 5, 760 lbs. 9
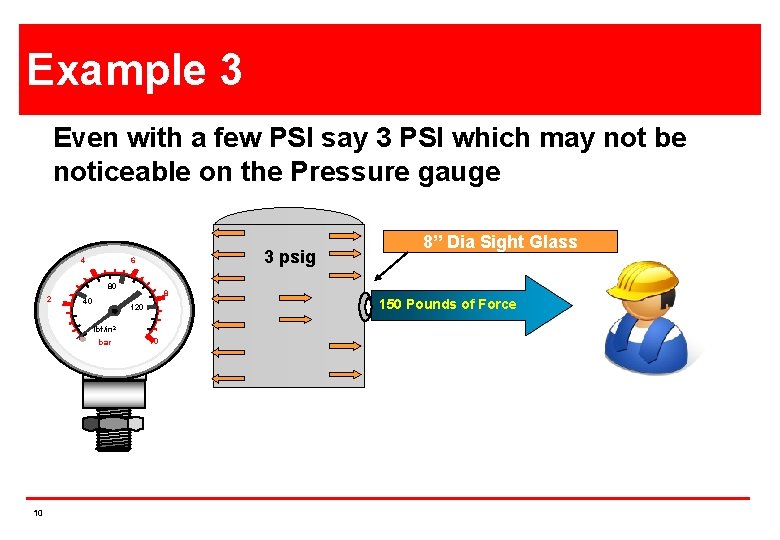
Example 3 Even with a few PSI say 3 PSI which may not be noticeable on the Pressure gauge 4 3 psig 6 80 2 40 8 120 lbf/in 2 bar 10 10 8”XDia Glass 12”Sight Square 150 Pounds of Force

BE AWARE! Can you get hurt with low pressure? ABSOLUTELY ! Never underestimate the potential of a low pressure or vacuum condition to cause damage. …. and be especially careful with large surfaces like manways. 1 psi may not even register on the gauge but it’s enough to send a hatch flying if the triclamp is removed and the gasket is stuck. 11

Low Pressure Hazard n This door had the equivalent of 1915 lbs of force on it. And at only 2. 8 psi. n n 12 The door weighs about 15 pounds much less than the 1915 lbs of force on it. When it came loose, it slammed open seriously injuring a person.

Over-Pressured Tank This tank was fitted with: § A high level alarm, which was § § § 13 accepted, and then forgotten A pressure control system, which was out of service A pressure relief valve which was found to be blocked …. so when the product was transferred into the tank, it over pressured until the roof ruptured, even though the pressure was only a few psi over hydrostatic
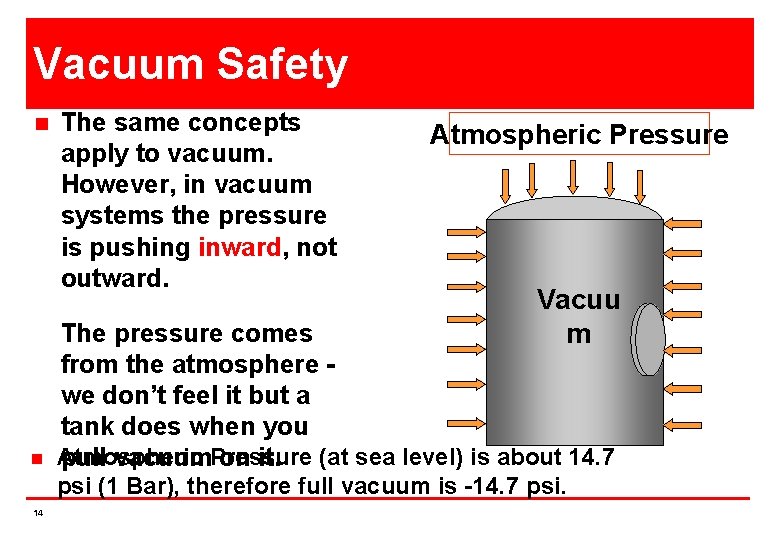
Vacuum Safety n n The same concepts apply to vacuum. However, in vacuum systems the pressure is pushing inward, not outward. Atmospheric Pressure Vacuu m The pressure comes from the atmosphere we don’t feel it but a tank does when you Atmospheric pull vacuum. Pressure on it. (at sea level) is about 14. 7 psi (1 Bar), therefore full vacuum is -14. 7 psi. 14

Vacuum Tank Hazards § When the pressure inside the vessel is lower than atmospheric pressure, the force acts inwards, with sometimes spectacular results…. § The tanker was being steam cleaned and, at the end of the job, the hatches were closed. With no vacuum breaker fitted, as the steam condensed, the tanker imploded…. . § Remember with a vacuum pulled, Hatches may appear stuck - venting to Equalise Pressure, eases hatch removal. Never pressurise in an effort to remove “Stuck Hatches” 15

Covered Vent n 16 This tank collapsed while being pumped out! Painters had covered the vent with plastic sheeting. The steel tank collapsed before the plastic sucked through.

Lack of Venting again n 17 This tank also collapsed while being pumped out!

Follow the Correct procedure when removing or opening vessel entry When removing sight glass to points Always open make chemical addition to water n vent valves if Possible in media tank 8 6 4 12 2 14 0 n Sight glass n n 18 10 bar 16 Always open vent valves if possible, to ensure Pressure Equalisation. Check the pressure gauge reading first, remember to eliminate “parallax error” from your reading. Ensure pressure has not been isolated at the regulator. Never Pressurise a vessel to break a seal, because at just a few PSI the subsequent pressure release will be detrimental to property or the person.
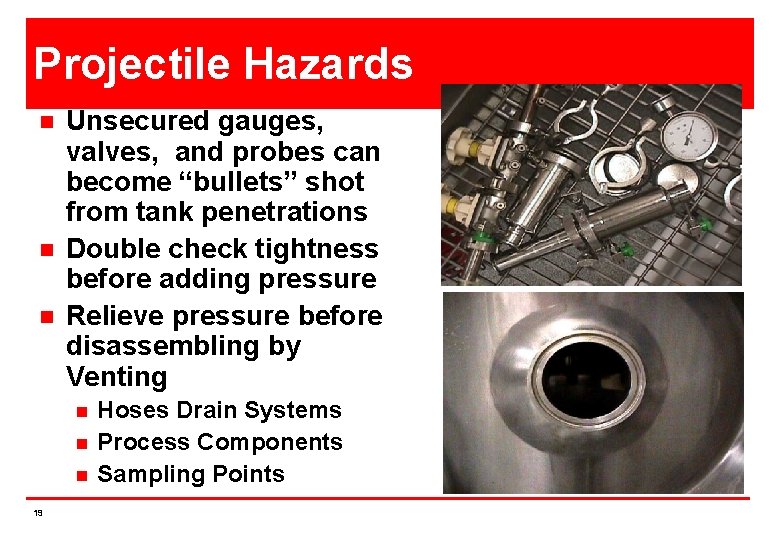
Projectile Hazards n n n Unsecured gauges, valves, and probes can become “bullets” shot from tank penetrations Double check tightness before adding pressure Relieve pressure before disassembling by Venting n n n 19 Hoses Drain Systems Process Components Sampling Points
Flailing Hoses n n n n 20 Pressurised Hose connection that fails will Flail. Double Check tri-clamp connection before adding pressure to hose. Routinely check the condition of hoses for any signs of bulging or failure. A whipping line can break bones and damage equipment, If the line gets free leave the area immediately and shut off flow to the line. Never attempt to grab a whipping Line or Hose. Flexible hosing should be kept as short as possible
Temperature, Burns and Scalds n n n 21 Hot WFI at 85 Degrees C Clean Steam at 145 Degrees C Both at Pressures between 6 Bar and 1. 2 Bar. High Risk of Scald injury from hot spray or leak. High risk of Burns from surface of hot pipe work or adjacent hot equipment.
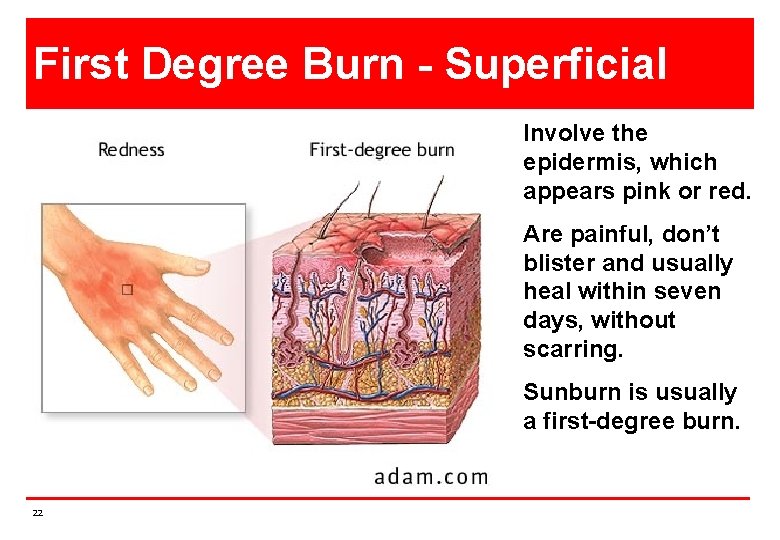
First Degree Burn - Superficial Involve the epidermis, which appears pink or red. Are painful, don’t blister and usually heal within seven days, without scarring. Sunburn is usually a first-degree burn. 22
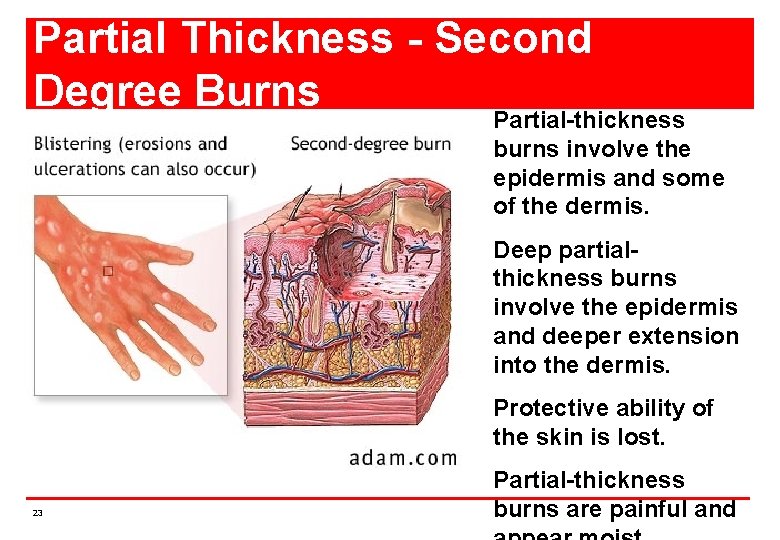
Partial Thickness - Second Degree Burns Partial-thickness burns involve the epidermis and some of the dermis. Deep partialthickness burns involve the epidermis and deeper extension into the dermis. Protective ability of the skin is lost. 23 Partial-thickness burns are painful and
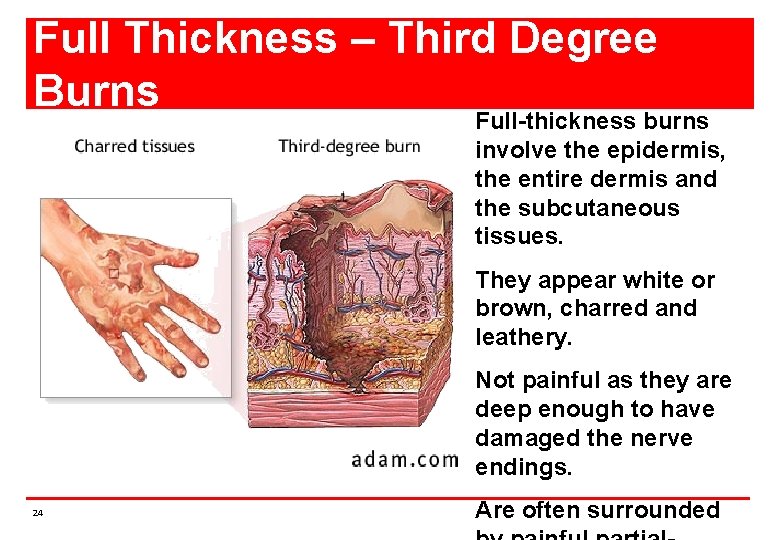
Full Thickness – Third Degree Burns Full-thickness burns involve the epidermis, the entire dermis and the subcutaneous tissues. They appear white or brown, charred and leathery. Not painful as they are deep enough to have damaged the nerve endings. 24 Are often surrounded

Incidents to take note of Case #1: n Case #2: Accident n Case #3: n Case #4: two Bar n 25 Tank Light Incident Production Support Sight Glass Operator Burn Incident Manway opening at less than

Case # 1: Tank Light Incident n n n 26 Technician was removing tank light (yellow arrow) Technician did not check pressure gauge first Start-up engineers had pressurized tank to 15 psi Light fixture blew off, stopped by pipe above. Sounded like shotgun Property damage only. Luck ‘prevented’ injury.

Case # 2: Production Support Sight Glass Accident Vent valve closed n n n Sight glass 27 n Technician was removing sight glass to make powder addition to water in Buffer Tank Technician did not check pressure gauge first Vent valve usually open, but had been left closed by others from prior activity; water addition created 18 psi inside tank Sight glass struck chin: 11
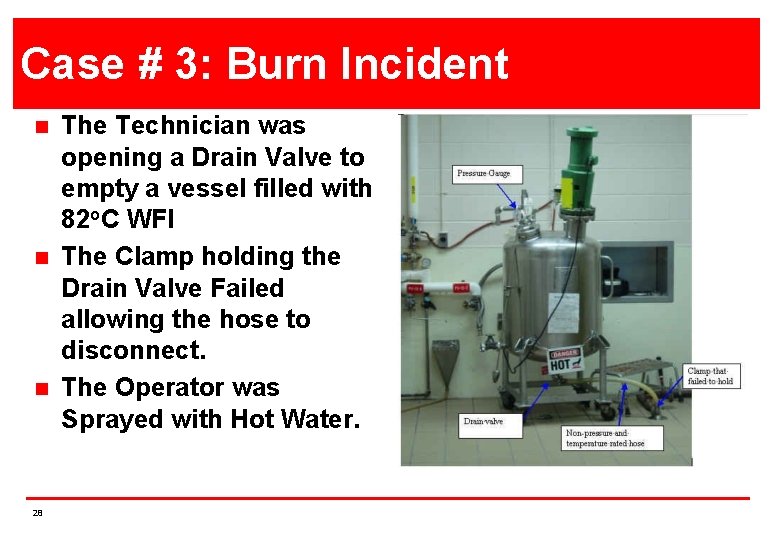
Case # 3: Burn Incident n n n 28 The Technician was opening a Drain Valve to empty a vessel filled with 82 o. C WFI The Clamp holding the Drain Valve Failed allowing the hose to disconnect. The Operator was Sprayed with Hot Water.
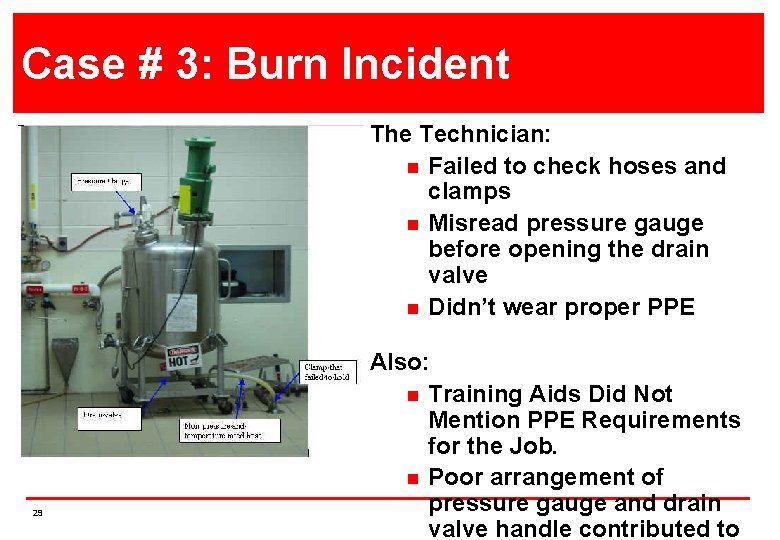
Case # 3: 2 Operator Burn Incident Case Burn Incident The Technician: n Failed to check hoses and clamps n Misread pressure gauge before opening the drain valve n Didn’t wear proper PPE 29 Also: n Training Aids Did Not Mention PPE Requirements for the Job. n Poor arrangement of pressure gauge and drain valve handle contributed to

Case # 4: Manway Opening at Less than Two Bar n n n 30 The pressure in the vessel had risen to 24. 7 PSI (1. 74 Barg) during a routine operation. Once the pressure reached 24. 7 PSI, the tri-clamp was unable to keep the manway in place and the manway blew open, causing the triclamp to hit and damage the ceiling above. Pressure acting on 12” Port (Surface Area = 113 Sq. Inches)

Case # 4: Manway Opening at Less than Two Bar Tri-clamp Deformation 31 Hole in ceiling where triclamp penetrated (6 ft up)

Case # 4: Manway Opening at Less than Two Bar n n Failure occurred at a vessel pressure <2. 0 Bar A vent valve was programmed to open when the pressure reached approximately 1. 8 Bar n n n 32 This valve did not open. Both of the clamp bolts were cut prior to use (To fit) The bolt that failed was cut shorter than the other one The Operator was unable to see how well the connection was made as dome nuts covered the thread of the bolts The Vessel was within normal operating pressures

Safety Systems n Tool Box Talks n n n Safe Plan of Action n n 33 Communications of Issues. Link Productivity with Safety and Quality. Incident communication and prevention. Learn from mistakes. Hazard review of the task. Involve all involved in the task. To be done on site while reviewing the hazard Where a Safe Plan is carried out – Quality and productivity follow suite

Schedule Based Project – the risk n n Serious Injury or incident will affect the project schedule. Loss time due to: n n n n 34 The Incident itself. Personal loss to individual and family. Incident investigation. Investigation by H. S. A or Garda. Closure of part of the project. Loss of moral. The list is endless…….

4) Validation n Introduction n What requires validation? n Pre Qualification Activities n Stages of Validation 35
What is Validation? Ronseal Definition § Proving with documented evidence that ‘Ronseal does exactly what it says on the tin!!’…. . 36 Sober Definition § The purpose of validation is to establish documented evidence which provides a high degree of assurance that premises, facilities, equipment or processes have been designed in accordance with the requirements of current Good Manufacturing Practice (c. GMP).
What Areas Require Validation? All systems or just c. GMP systems § § § § § 37 Analytical and Quality Procedures Instruments Critical Support Systems (e. g. HVAC, WFI) Raw Materials and Packaging Equipment Design, Installation and Operation Facility Design, Installation and Operation Maintenance Systems Manufacturing Processes Product Design and Development Control Systems

Qualification Activities n n n 38 GMP systems and equipment qualified through documented challenge testing to verify that design intent and user requirements have been met GMP definition includes systems and equipment used in manufacture, control, monitoring, storage and analysis of pharmaceutical product Where appropriate, documented successful prequalification activities will be cited and referenced in lieu of repeating execution steps in IQ (Installation Qualification) and OQ (Operational Qualification) IQ and OQ required for all GMP systems PQ for critical utility and manufacturing equipment
Where do you start ? 39

Validation Master Plan (VMP) § § Generally prepared for the start-up of a large project Serves as both a guide to the Client and is used as a review document for regulatory agencies Develops concurrently with the project - a living document Purpose of a VMP Ø Ø Ø 40 To briefly describe why, what, by whom, how and when the validation is to be carried out. Provide up-to-date information about the actual state of affairs relating to validation Demonstrate the companies commitment to carry out validation

Pre-Qualification Activities Purpose is to establish system / equipment installation and functionality prior to commencing formal qualification testing n Steps include: n Enhanced Design Review n Factory Acceptance Testing (FAT) n Commissioning n Site Acceptance Testing (SAT) n 41
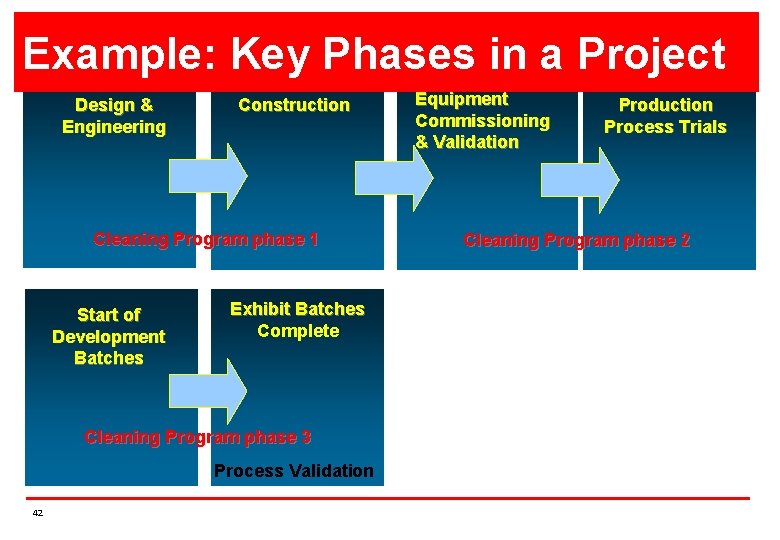
Example: Key Phases in a Project Design & Engineering Construction Cleaning Program phase 1 Start of Development Batches Exhibit Batches Complete Cleaning Program phase 3 Process Validation 42 Equipment Commissioning & Validation Production Process Trials Cleaning Program phase 2
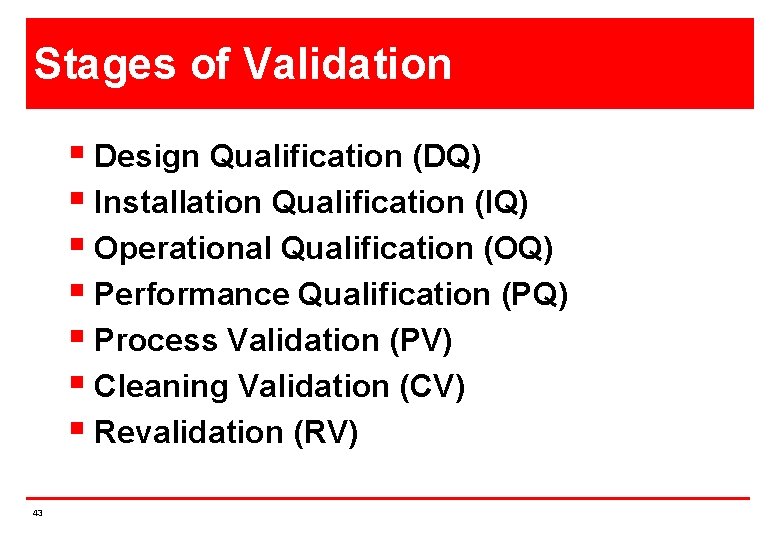
Stages of Validation § Design Qualification (DQ) § Installation Qualification (IQ) § Operational Qualification (OQ) § Performance Qualification (PQ) § Process Validation (PV) § Cleaning Validation (CV) § Revalidation (RV) 43

Design Qualification (DQ) § Provides documented evidence that the design/ quotation acceptance satisfies the approved User Requirement Specification (URS). § Documenting that we are getting what we asked for in our design i. e. Miele Dish Washer capable of washing at temps from 25 to 80 Deg C, with air drying on a range of dirty soils with detergent capability 44

Installation Qualification (IQ) § Provides documented verification that all key aspects of the installation adhere to design specification, regulatory and statutory codes and manufacturers recommendations. § Think of a dish washer at home. Checking vendor is installing it correctly as per the recommendations…Consider does this always go well? !! 45

Operational Qualification (OQ) § Provides documented verification that the system and sub-systems perform as intended throughout all anticipated operating ranges. § Think of dish washer again - Proving that it is capable of running all of its cycles with process water in your house and a range of detergents …drainability, spray coverage etc 46

Performance Qualification (PQ) § Provides documented evidence that a GMP utility service or process when operated or carried out within defined parameters will consistently meet pre-determined acceptance criteria. PQ will be performed on those systems or processes that require performance data for verifying properation. § Proving that with Production materials, i. e. dirty 47 plates, that it will consistently clean to its pre defined criteria i. e. clean and dry within an allocated time
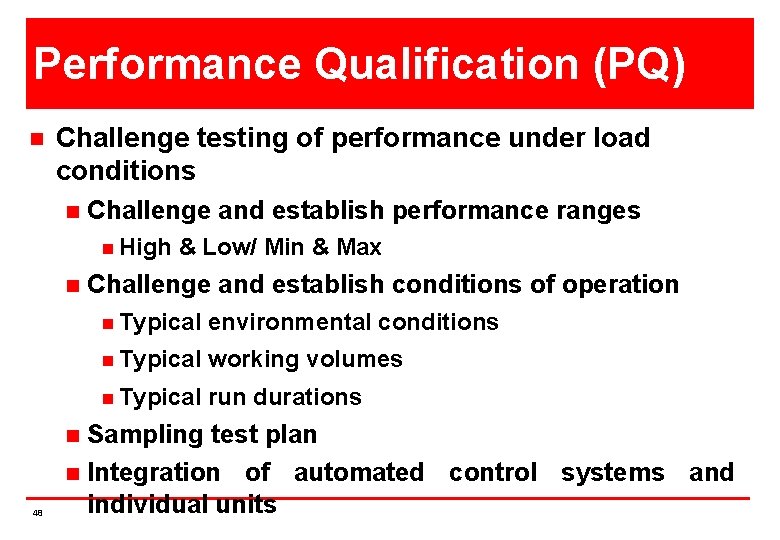
Performance Qualification (PQ) n Challenge testing of performance under load conditions n Challenge and establish performance ranges n High n Challenge and establish conditions of operation n Typical environmental conditions n Typical working volumes n Typical run durations Sampling test plan n Integration of automated control systems and individual units n 48 & Low/ Min & Max
Media Simulation § Important step of aseptic processing § Demonstrates that the process, equipment, § 49 people and utilities when operating together are capable of reliably producing sterile product. Bacteria friendly food (Media) is processed through all of the equipment as a challenge. A simulation is likely to include the following steps n Media compounding n Sterilisation of the media by filtration

Cleaning Validation (CV) 50 n Program designed to ensure that equipment can be cleaned to a pre-defined cleaning acceptance criteria to ensure that any carryover of product does not affect the strength, purity, identity, quality or safety of the subsequent product n Limits for cleaning based on toxiciology of product in body, and consider the risk of transferring small amounts of 1 product into another.
Cleaning Validation (CV) Cleaning Cycles n n n 51 Focus on efficacy of set parameters Validate on “worst case” basis, and provide added level of assurance of efficacy in actual operation Execution of process parameters monitored with each execution Cleaning validation will address operations performed between campaigns of different products Cleaning validation will address operations performed between the manufacturing of batches of the same product
Process Validation (PV) Irrespective of product, Process Validation is preceded by a formal, documented development phase: n n n n 52 Run media runs and hold studies Complete transfer / validation of test methods Challenge process and manufacturing equipment set points, ranges and durations Evaluate efficacy of equipment cleaning processes Re-establish in-process product hold intervals Finalization and approval of manufacturing process and batch record Evaluate comparability of development batches to licensed material
Process Validation (PV) General Requirements of Process Validation: n n n 53 Can be based on successful validation and approval of the products at other licensed sites Minimum of three production batches Evaluate manufacturing operations using qualified facilities and equipment train, under typical processing conditions Confirm stability of product through established expiry period Demonstrate reproducible manufacturing process capable of meeting pre-determined quality attributes Verify shipping conditions and product impact resulting from shipment to regional packaging facility
Process Validation (PV) Example: Drug Product Manufacturing Validation will focus on: n Demonstrating ability to maintain established environmental and aseptic conditions throughout the manufacturing process Maintaining all equipment settings within established ranges Confirm performance of the formulation process n Evaluation of product transfer steps: n n Ø Filling n n 54 line: Set up Speed Filling duration Consistency across batch Capping system operation: Crimping Vial track

Revalidation (RV) § The action of providing documented evidence that equipment and ancillary systems continue to operate within pre-determined and specified ranges and continue to meet user needs as established through validation testing. This is accomplished by performing planned Revalidation testing, performing Periodic Review and, where deemed necessary, Revalidation testing as a result of the conclusion from the Periodic Review performed. § 55 Ensuring on a periodic basis that the dish Washer is still working. The worst case washing cycle would be run and documented i. e. does you dish washer work consistently !!
Documentation Practices Types of Documents n n n 56 Procedures/ Protocols for DQ/IQ/OQ/PQ Cleaning and Process Validation Reports summarising the validation studies providing conclusion to validated status of equipment Deviations : raised when certain tests acceptance criteria cannot be met. These need to be investigated and closed out prior to Validation summary report approval.
- Slides: 56