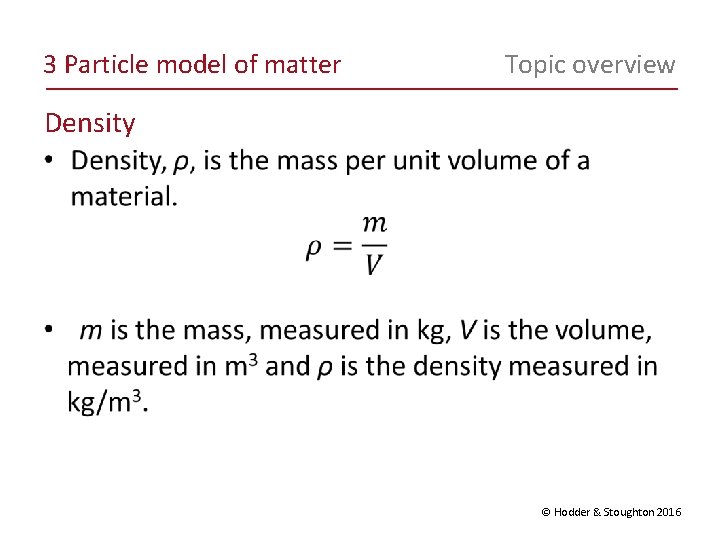
3 Particle model of matter Topic overview Density
- Slides: 12

3 Particle model of matter Topic overview Density © Hodder & Stoughton 2016

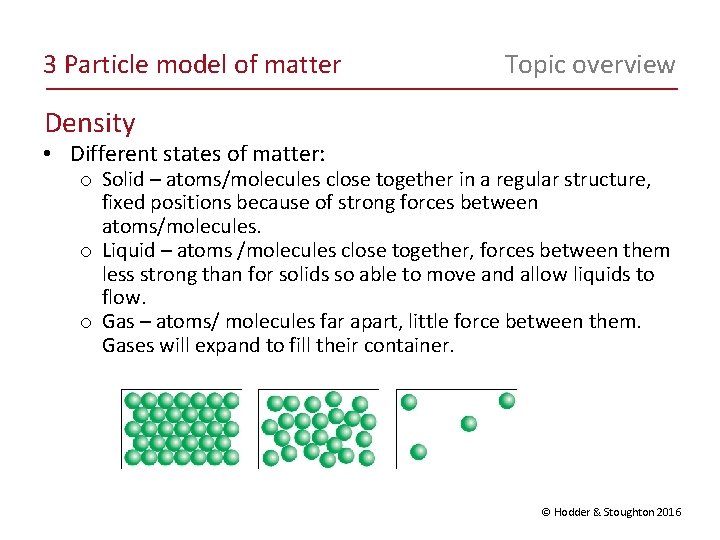
3 Particle model of matter Topic overview Density • Different states of matter: o Solid – atoms/molecules close together in a regular structure, fixed positions because of strong forces between atoms/molecules. o Liquid – atoms /molecules close together, forces between them less strong than for solids so able to move and allow liquids to flow. o Gas – atoms/ molecules far apart, little force between them. Gases will expand to fill their container. © Hodder & Stoughton 2016

3 Particle model of matter Topic overview Density • Density of solids and liquids is greater than gas. • Different materials have different densities because the atoms/molecules have different masses. • Most solid materials are more dense than their liquid; water is one exception. © Hodder & Stoughton 2016

3 Particle model of matter Topic overview Changes of state • A change of state is when a material changes from solid to liquid, liquid to gas or solid to gas. • In these changes the total mass stays constant. • The changes are physical changes and are reversible. o Melting – change from solid to liquid state. o Freezing – change from liquid to sold state. o Boiling/evaporation – change from liquid to gas state. o Condensation – change from gas to liquid state. o Sublimation – change from solid to gas state. • A change of state is a result of a change in internal energy of the material. © Hodder & Stoughton 2016

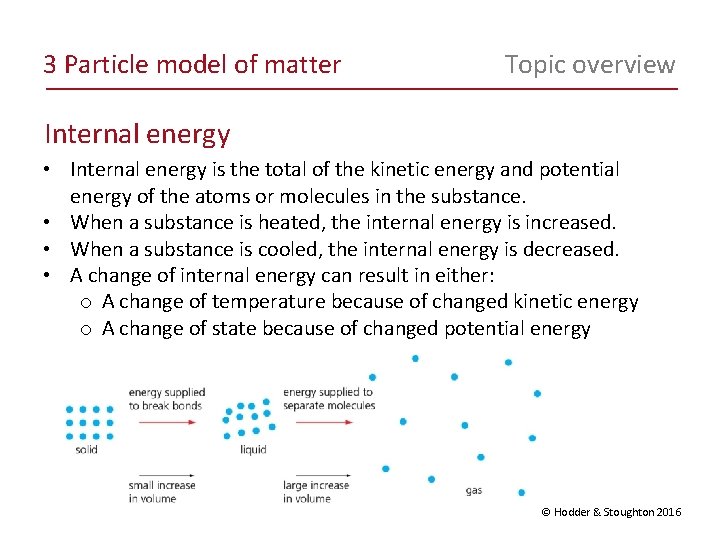
3 Particle model of matter Topic overview Internal energy • Internal energy is the total of the kinetic energy and potential energy of the atoms or molecules in the substance. • When a substance is heated, the internal energy is increased. • When a substance is cooled, the internal energy is decreased. • A change of internal energy can result in either: o A change of temperature because of changed kinetic energy o A change of state because of changed potential energy © Hodder & Stoughton 2016

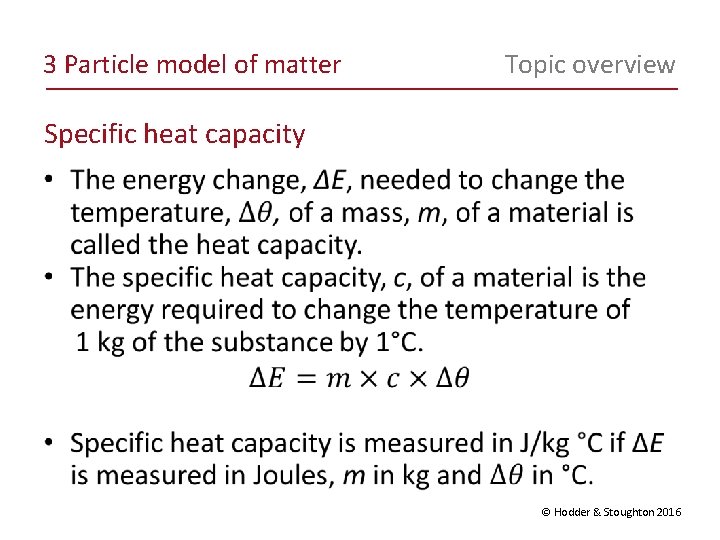
3 Particle model of matter Topic overview Specific heat capacity © Hodder & Stoughton 2016

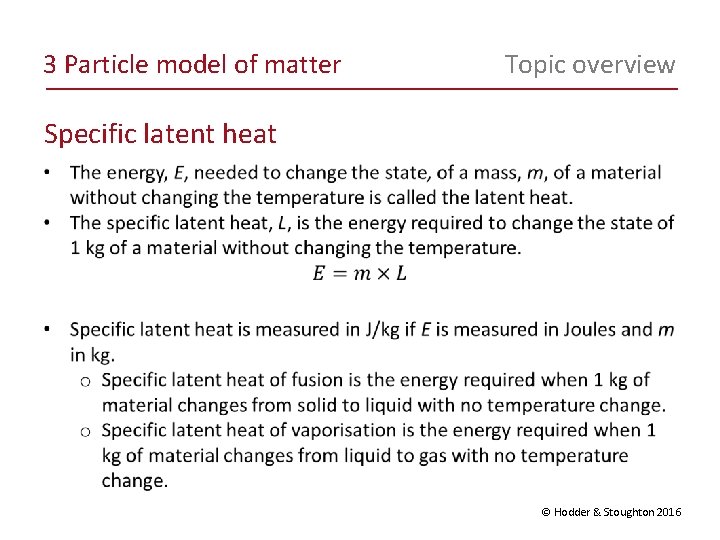
3 Particle model of matter Topic overview Specific latent heat © Hodder & Stoughton 2016

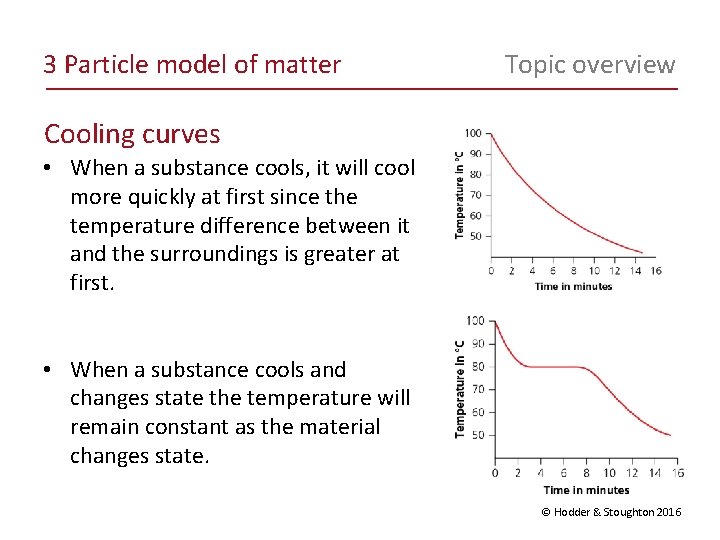
3 Particle model of matter Topic overview Cooling curves • When a substance cools, it will cool more quickly at first since the temperature difference between it and the surroundings is greater at first. • When a substance cools and changes state the temperature will remain constant as the material changes state. © Hodder & Stoughton 2016

3 Particle model of matter Topic overview Particle motion • The particle model or kinetic theory of gases assumes that atoms/molecules in a gas: o are in constant random motion. o collide with each other and the walls of the container without loss of kinetic energy. o cause a force at right angles to the surface of the container at each collision. • Temperature is related to the average kinetic energy of atoms/molecules. • An increase in the average kinetic energy is an increase in temperature. © Hodder & Stoughton 2016

3 Particle model of matter Topic overview Pressure in a gas • The atoms/molecules of a gas at a higher temperature have more kinetic energy and so are moving faster. • The faster and the more frequent the collisions of atoms/molecules with the walls of their container, the greater the force on the walls. • For a constant volume, a greater force on the walls results in an increase in pressure. © Hodder & Stoughton 2016

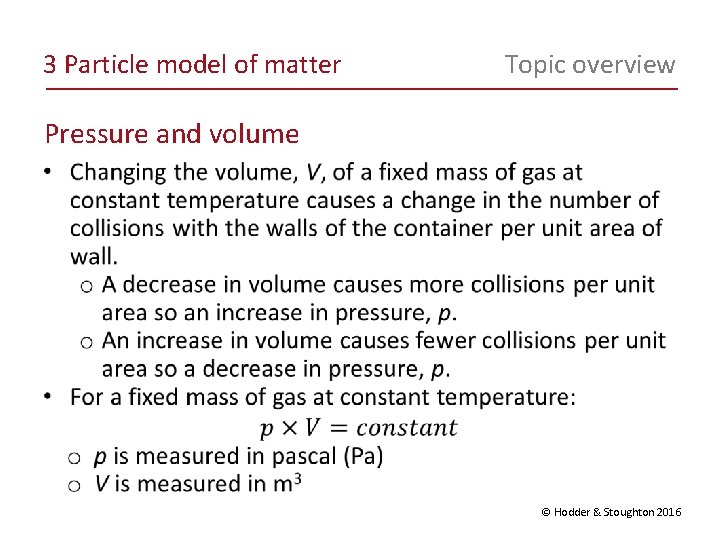
3 Particle model of matter Topic overview Pressure and volume © Hodder & Stoughton 2016

3 Particle model of matter Topic overview Work done on a gas • When work is done on a gas, e. g. compressing a gas by using a pump, the average k. e. of the atoms/molecules increases. Work done = force x distance moved in direction of force • When the average kinetic energy of the atoms/molecules increases, the temperature of the gas rises. © Hodder & Stoughton 2016