3 HEMOGLOBIN STRUCTURE FUNCTION IRON METABOLISM Prof Sherif

3. HEMOGLOBIN: STRUCTURE, FUNCTION & IRON METABOLISM. Prof. Sherif W. Mansour Physiology dpt. , Mutah School of medicine 2020 -2021
Haemoglobin • Definition: It is the principal constitute (33% ) of RBCs. It is a red pigment which gives the blood its red colour. • Normal levels: - Infant at birth 20 gm/dl (as the newborn has more RBCs due to relative intrauterine ischemia). -Infant after one week 15. 5 gm/dl - Children (3 -12 years) =11 -14 gm/dl (due to gradual destruction of RBCs) - Adult male 13 -18 gm/dl - Adult female 12 -16 gm/dl.

• Structure: -It is formed of : (1) Globin: 2 pairs of polypeptide chains (2 α and 2 B) (2) 4 Haem: each is an iron-protoporphyrin and formed by: - 2 succinyl – Co. A + 2 glycine → one pyrrole ring. - 4 pyrrole rings→ protoporphyrin - protoporphyrin + Fe++ → haem. -So, it is made of 4 subunits each of them is formed from one Haem and one polypeptide chain. • Functions: - Carriage of O 2 & CO 2 - Strong buffer system.
• Reactions of Hb: 1. Oxyhemoglobin: O 2 bind with iron in ferrous state so it is called oxygenation not oxidation. This binding affected by p. H, temperature and 2, 3 -diphosphoglycerate in RBCs. 2. Met Hb: strong oxidation by certain drugs or oxidizing agents → ferric state which not carry O 2 → dusky colouration of skin like cyanosis (normally, Met. Hb doesn’t exceed 0. 5% due to the activity of NADH-Met. Hb-reductase enzyme in the RBCs which converts it back to normal Hb). 3. Carboxy Hb: carbon monoxide is a toxic gas and attached to Fe++ in high affinity (210 times as O 2). This part attached to CO doesn’t carry O 2 and the remaining part of Hb, which carries O 2 doesn’t give its O 2 to the tissue. 4. Carbamino Hb: normally Co 2 attached to the globin part of Hb.

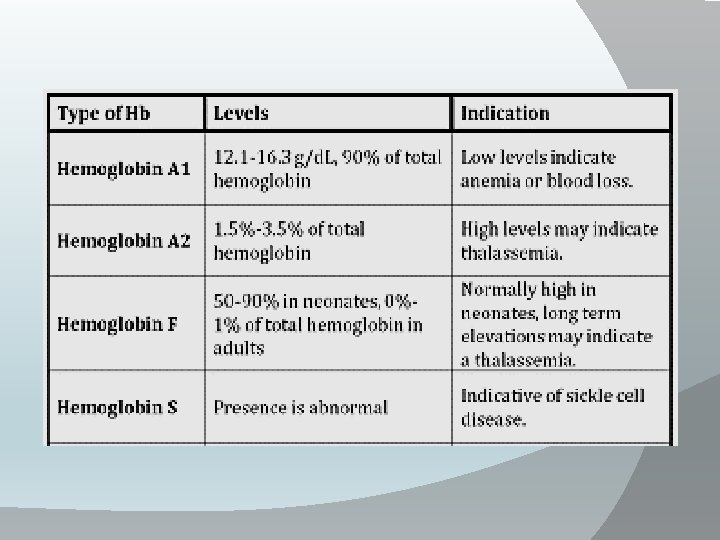
• Types of Hb: 1 -Adult (Hb. A): contain 2 α chain (each is consisted of 141 amino acids) and 2 B chain (146 amino acids). (97. 5% of adult Hb. ) 2 -Hb. A 2: contain 2α chains and 2 delta (146 amino acids) chains which differ from Bchains in the terminal 10 A. A. 3 -Fetal Hb (Hb. F): -It is the type of Hb in the human fetus then it is usually replaced by adult Hb after ɤ birth. -It contain 2α and 2 gamma(146 amino acids) chains which differ from Bchains in 37 A. A. -It has high affinity for O 2 and less to 2, 3 di-phosphoglycerate , So this facilitate movement of O 2 from maternal circulation to the fetus. 4 -Glycosylated Hb: (3 -7% of Hb) glucose is attached to terminal valine amino acid in B-chain. This value increases in cases of uncontrolled diabetes mellitus. 5 -Hb. S : It is abnormal type of Hb due to congenital abnormality of B-globin in which valine amino acid present instead of normal glutamic acid at position 6 of B-chain → hemoglobin-S which causes sickle cell anaemia.

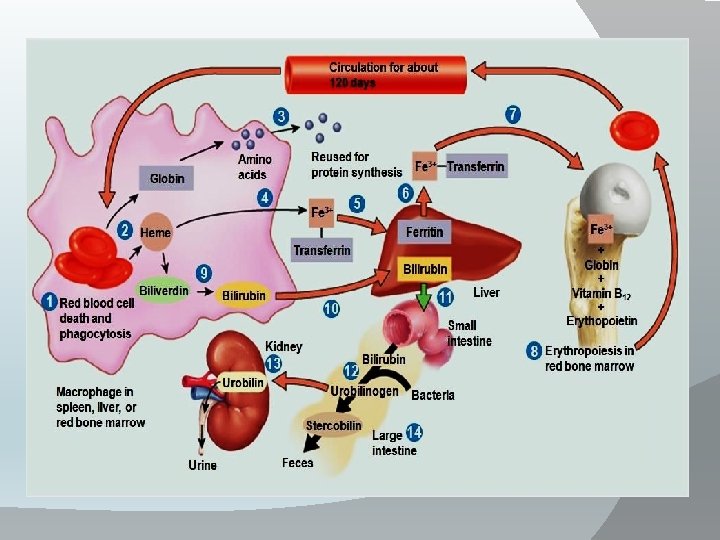
Destruction of RBCs: • The life span of RBCs is 120 days , old RBCs destroyed in narrow capillaries and spleen →Hb released and splits by the cells of the reticuloendothelial system into globin & haem. • Globin used in protein metabolism. • Haem loss its iron to be stored as ferritin. • The protoporphyrin part of haem → bile pigments (biliverdin which reduced into bilirubin) which conjugated and secreted by the liver with bile to small intestine to be excreted into stool and some reabsorbed to blood and secreted in urine.
Thank You

Thank You
- Slides: 10