3 GROUP CATIONS Fe 3 Al 3 Cr




- Slides: 10

3. GROUP CATIONS Fe 3+, Al 3+, Cr 3+, Ni 2+, Co 2+, Mn 2+, Zn 2+

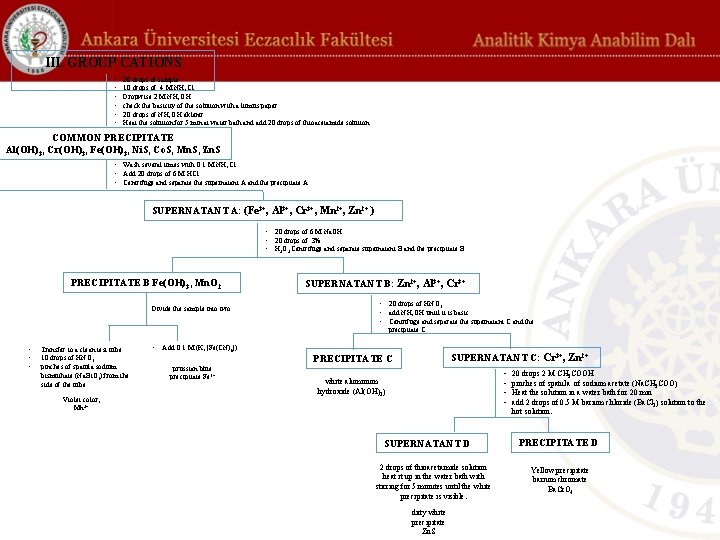
These cations precipitate as sulfides and hydroxides when reacted with hydrogen sulfide (H 2 S) or thioacetamide solution in basic medium buffered with NH 4 OH – NH 4 Cl. These cations do not precipitate with the reagents used for precipitation of the group I and II. cations. Group III cations are divided into two: 1) Iron Group (Group 3 A): Fe 3+, Al 3+, Cr 3+ (precipitates in the form of hydroxides). 2) Zinc Group (Group 3 B): Ni 2+, Co 2+, Mn 2+, Zn 2+ (precipitates in the form of sulfides).

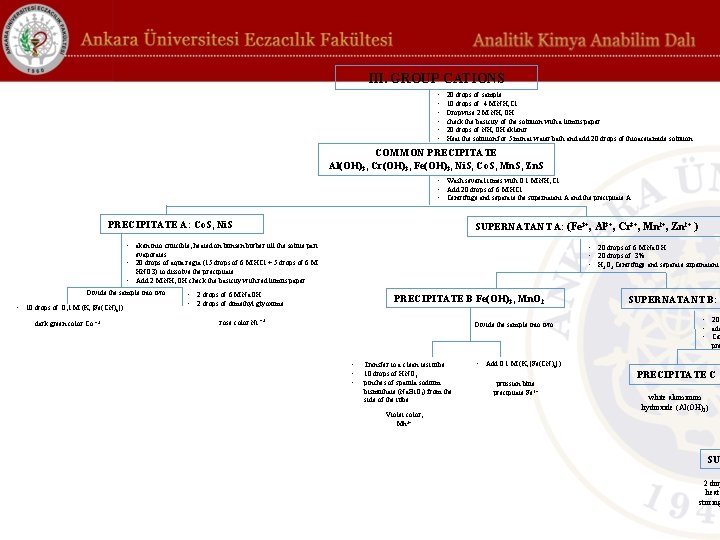
Systematic analysis of group III cations: 20 drops of sample containing Fe 3+, Al 3+, Cr 3+, Ni 2+, Co 2+, Mn 2+, Zn 2+ taken into a tube from the sample. Add 10 drops of 4 M ammonium chloride (NH 4 Cl) solution and 10 drops of 2 M ammonium hydroxide (NH 4 OH, %33) solution dropwise to the sample and check the basicity of the solution with a litmus paper. Then add 20 drops of NH 4 OH solution and Fe 3+, Al 3+ and Cr 3+ precipitate as their hydroxides. Heat the solution for 5 min at water bath and add 20 drops of thioacetamide solution (11%, w/v) and observe precipitates of sulfides of Ni 2+, Co 2+, Zn 2+ and Mn 2+ (COMMON PRECIPITATE) This precipitate contains Al(OH)3, Cr(OH)3, Fe(OH)3, Ni. S, Co. S, Mn. S, and Zn. S and it is washed several times with 0. 1 M NH 4 Cl.

Precipitate A (Co. S ve Ni. S): Precipitate A is taken into cruicible, heated on bunsen burber till the solute part evaporates if exist, and add 20 drops of aqua regia (15 drops of 6 M HCl + 5 drops of 6 M HNO 3) to dissolve the precipitate. Add from NH 4 OH (%33) solution until it is basic (check with red litmus paper). Divide the solution into two parts. To the first part, add 10 drops of 0. 1 M potassium ferricyanide (K 4[Fe(CN)6]) solution and the dark green color is followed, which is the precipitate of cobalt ferricyanide (Co 2[Fe(CN)6]). This shows the presence of Co+2. To the second part, add 2 drops of 6 M Na. OH and 2 drops of dimethyl glyoxime (%1 in 96% alcohol), the rose color shows the presence of Ni+2.

Supernatant A (Fe 3+, Al 3+, Cr 3+, Mn 2+, Zn 2+ ): Add 20 drops of 6 M Na. OH and 20 drops of 3% H 2 O 2 (v/v) are added to the supernatant. Centrifuge and separate supernatant B and the precipitate B. Transfer precipitate B to a clean test tube, add 10 drops of HNO 3 (37. 5%) and pinches of spatula sodium bismuthate (Na. Bi. O 3) from the side of the tube. The color of violet proves the presence of Mn 2+. Add 0. 1 M K 4[Fe(CN)6] to the precipitate B, the prussian blue precipitate proves the presence of Fe 3+.

Supernatant B (Zn 2+, Al 3+, Cr 3+): Add 20 drops of HNO 3 and NH 4 OH to the solution until it is basic. Centrifuge and separate the supernatant C and the precipitate C Precipitate C is white aluminum hydroxide (Al(OH)3). The yellow color of the supernatant C, which contains Cr 3+ and Zn 2+, indicates the presence of Cr 3+. Add 20 drops of 2 M CH 3 COOH to the supernatant C to make the medium acidic. Add pinches of spatula of sodium acetate (Na. CH 3 COO). Heat the solution in a water bath for 20 min and add 2 drops of 0. 5 M barium chloride (Ba. Cl 2) solution to the hot solution. The formed yellow precipitate (precipitate D) is barium chromate (Ba. Cr. O 4). Add 2 drops of thioacetamide solution (11% w/v) to the supernatant D and heat it up in the water bath with stirring for 5 minutes until the white precipitate is visible. The formation of dirty white zinc sulfide (Zn. S) shows the presence of Zn 2+.


III. GROUP CATIONS • • • 20 drops of sample 10 drops of 4 M NH 4 Cl Dropwise 2 M NH 4 OH check the basicity of the solution with a litmus paper. 20 drops of NH 4 OH eklenir. Heat the solution for 5 min at water bath and add 20 drops of thioacetamide solution COMMON PRECIPITATE Al(OH)3, Cr(OH)3, Fe(OH)3, Ni. S, Co. S, Mn. S, Zn. S • Wash several times with 0. 1 M NH 4 Cl • Add 20 drops of 6 M HCl • Centrifuge and separate the supernatant A and the precipitate A. PRECIPITATE A: Co. S, Ni. S SUPERNATANT A: (Fe 3+, Al 3+, Cr 3+, Mn 2+, Zn 2+ ) • aken into cruicible, heated on bunsen burber till the solute part • 20 drops of 6 M Na. OH • 20 drops of 3% • H 2 O 2 Centrifuge and separate supernatant evaporates • 20 drops of aqua regia (15 drops of 6 M HCl + 5 drops of 6 M HNO 3) to dissolve the precipitate. • Add 2 M NH 4 OH check the basicity with red litmus paper. Divide the sample into two • 10 drops of 0, 1 M (K 4[Fe(CN)6]) dark green color Co +2 • 2 drops of 6 M Na. OH • 2 drops of dimethyl glyoxime PRECIPITATE B Fe(OH) 3, Mn. O 2 rose color Ni +2 Divide the sample into two SUPERNATANT B: • 20 • add • Ce pre • • • Transfer to a clean test tube 10 drops of HNO 3 pinches of spatula sodium bismuthate (Na. Bi. O 3) from the side of the tube Violet color, Mn 2+ • Add 0. 1 M (K 4[Fe(CN)6]) prussian blue precipitate Fe 3+ PRECIPITATE C white aluminum hydroxide (Al(OH)3) SU 2 dro heat stirring

III. GROUP CATIONS • • • 20 drops of sample 10 drops of 4 M NH 4 Cl Dropwise 2 M NH 4 OH check the basicity of the solution with a litmus paper. 20 drops of NH 4 OH eklenir. Heat the solution for 5 min at water bath and add 20 drops of thioacetamide solution COMMON PRECIPITATE Al(OH)3, Cr(OH)3, Fe(OH)3, Ni. S, Co. S, Mn. S, Zn. S • Wash several times with 0. 1 M NH 4 Cl • Add 20 drops of 6 M HCl • Centrifuge and separate the supernatant A and the precipitate A. SUPERNATANT A: (Fe 3+, Al 3+, Cr 3+, Mn 2+, Zn 2+ ) • 20 drops of 6 M Na. OH • 20 drops of 3% • H 2 O 2 Centrifuge and separate supernatant B and the precipitate B. PRECIPITATE B Fe(OH) 3, Mn. O 2 Divide the sample into two SUPERNATANT B: Zn 2+, Al 3+, Cr 3+ • 20 drops of HNO 3 • add NH 4 OH until it is basic. • Centrifuge and separate the supernatant C and the precipitate C • • • Transfer to a clean test tube 10 drops of HNO 3 pinches of spatula sodium bismuthate (Na. Bi. O 3) from the side of the tube Violet color, Mn 2+ • Add 0. 1 M (K 4[Fe(CN)6]) prussian blue precipitate Fe 3+ SUPERNATANT C: Cr 3+, Zn 2+ PRECIPITATE C • • white aluminum hydroxide (Al(OH)3) SUPERNATANT D 2 drops of thioacetamide solution heat it up in the water bath with stirring for 5 minutes until the white precipitate is visible. dirty white precipitate Zn. S 20 drops 2 M CH 3 COOH pinches of spatula of sodium acetate (Na. CH 3 COO) Heat the solution in a water bath for 20 min add 2 drops of 0. 5 M barium chloride (Ba. Cl 2) solution to the hot solution. PRECIPITATE D Yellow precipitate barium chromate Ba. Cr. O 4

When excess NH 3 is added to the starting solution, red brown color indicates the presence of the Fe 3+, and/or colorless and gelatinous precipitate indicates the presence of Al 3+.