3 GAS CHROMATOGRAPHYGC 7 th lecture GC is














- Slides: 16

3. GAS CHROMATOGRAPHY(GC): 7 th lecture GC is currently one of the most popular methods for separating and analyzing compounds. GC can be applied to the separation of any compound that is either naturally volatile (i. e. , readily goes into the gas phase) or can be converted to a volatile derivative. Principle of separation: different adsorption or solubility of the components of the mixture

Stationary phase: adsorbent (GSC)or wetted carrier material (adsorbent coated by a liquid layer)( GLC ) Liquid layer: non-volatile liquids, macromolecular products A simple GC system consists of: 1. Gas source (with pressure and flow regulators) 2. Injector or sample application system (sample inlet) 3. Chromatographic column (with oven for temperature control) 4. Detector 5. computer or recorder

There are two main types of columns used in GC: 1 -Packed columns large sample capacity preparative work 2 -Capillary (open-tubular) columns higher efficiency smaller sample size analytical applications

Carrier gas: He (common), N 2, H 2 (MUST BE INERT) Flow = 25 -150 m. L/min packed column Flow = 1 -25 m. L/min open tubular column Oven: 0 -400 °C ( average boiling point of sample) common adsorbents include: • alumina • silica • active carbon


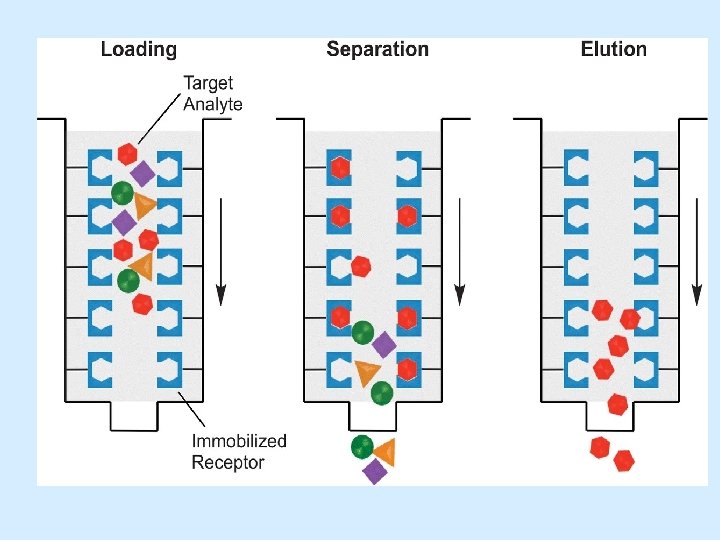
4. AFFINITY CHROMATOGRAPHY • The principle of affinity chromatography is that the stationary phase consists of a support medium (e. g. cellulose beads) on which the substrate has been bound covalently. • As the mixture of proteins is passed through the chromatography column, those proteins that have a binding site for the immobilised substrate will bind to the stationary phase, while all other proteins will be eluted in the void volume of the column.


• A protein mixture in a suitable buffer solution is passed down the column and a protein with sufficient affinity for the bound ligands is retarded and may later be eluted in a purified state by a change in ionic strength Or PH of the column buffer. • Ligands such as amino , hydroxyl, carbonyl and thio groups located to serve as ligand binding sites. • The ligand/ molecule can be dissociated by changing the PH

Uses : -production of vaccines. -Nucleic acid purification in genetic engineering. -Separation of enzymes and proteins. Advantages: 1 -high specifity 2 -high degree of purity Disadvantages : 1 -expensive ligands 2 -leakage of ligands 3 -relatively low productivity 4 -degradation of the solid support

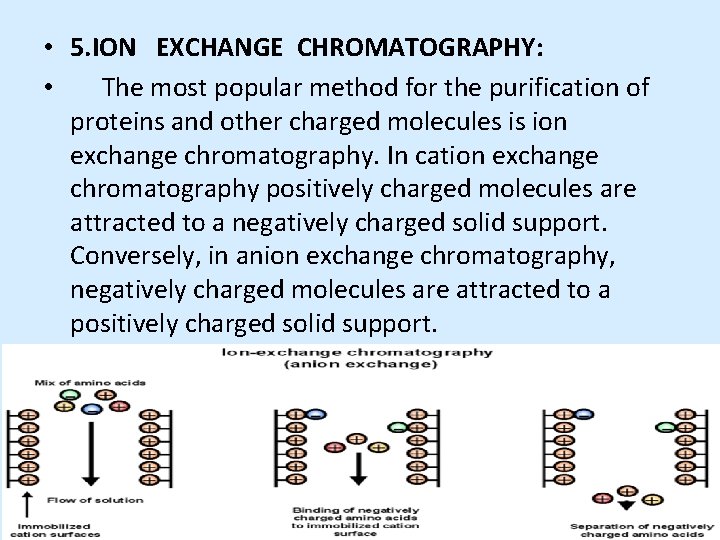
• 5. ION EXCHANGE CHROMATOGRAPHY: • The most popular method for the purification of proteins and other charged molecules is ion exchange chromatography. In cation exchange chromatography positively charged molecules are attracted to a negatively charged solid support. Conversely, in anion exchange chromatography, negatively charged molecules are attracted to a positively charged solid support.

• Mechanism • To optimize binding of all charged molecules, the mobile phase is generally a low to medium conductivity (i. e. , low to medium salt concentration) solution. The adsorption of the molecules to the solid support is driven by the ionic interaction between the oppositely charged ionic groups in the sample molecule and in the functional ligand on the support. • The strength of the interaction is determined by the number and location of the charges on the molecule and on the functional group. By increasing the salt concentration (generally by using a linear salt gradient) the molecules with the weakest ionic interactions start to elute from the column first.

• 6. ELECTROPHORESIS : • Electrophoresis may be the main technique for molecular separation in today's cell biology laboratory. Because it is such a powerful technique, and yet reasonably easy and inexpensive, it has become common. Electrophoresis can be one dimensional (i. e. one plane of separation) or two dimensional.


• One dimensional electrophoresis is used for most routine protein and nucleic acid separations. Two dimensional separation of proteins is used for finger printing. • Electrophoresis is a method for separation and analysis of macromolecules (DNA, RNA and proteins) and their fragments, based on their size and charge.

• It is used in clinical chemistry to separate proteins by charge and/or size and in biochemistry and molecular biology to separate a mixed population of DNA and RNA fragments by length, to estimate the size of DNA and RNA fragments or to separate proteins by charge.

• Nucleic acid molecules are separated by applying an electric field to move the negatively charged molecules through a matrix of agarose or other substances. Shorter molecules move faster and migrate farther than longer ones because shorter molecules migrate more easily through the pores of the gel. This phenomenon is called (sieving). Proteins are separated by charge in agarose • because the pores of the gel are too large to sieve proteins.