3 dTransition Elements Dr K Leena Assistant Professor







- Slides: 17

3 d-Transition Elements Dr. K. Leena, Assistant Professor & Head, Department of Nanoscience , Sarah Tucker college, Tirunelveli - 7

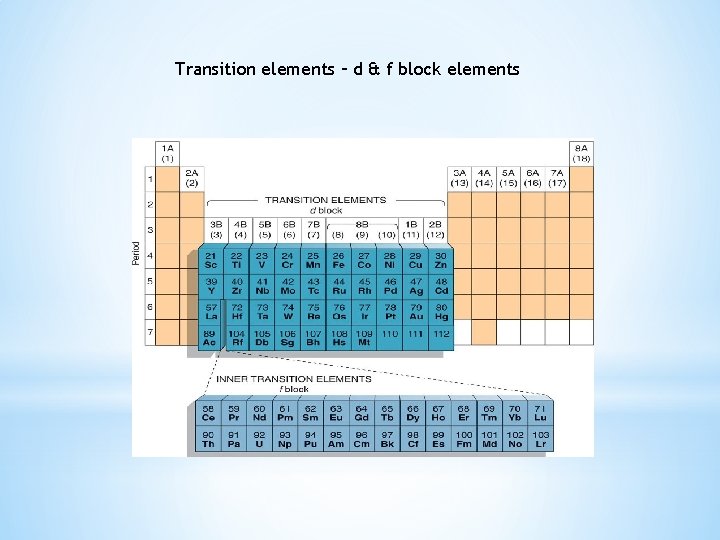
Transition elements – d & f block elements

Properties of the Transition Metals All transition metals are metals, whereas main-group elements in each period change from metal to nonmetal. Many transition metal compounds are colored and paramagnetic, whereas most main-group ionic compounds are colorless and diamagnetic. The properties of transition metal compounds are related to the electron configuration of the metal ion.

3 d – transition series metals.

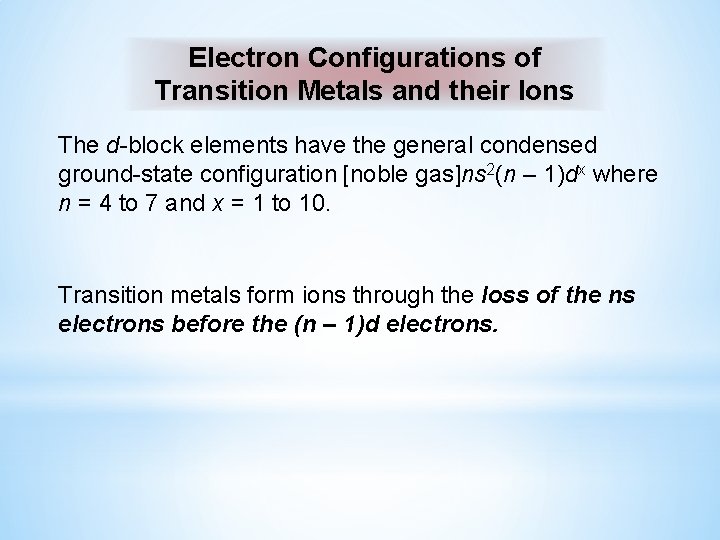
Electron Configurations of Transition Metals and their Ions The d-block elements have the general condensed ground-state configuration [noble gas]ns 2(n – 1)dx where n = 4 to 7 and x = 1 to 10. Transition metals form ions through the loss of the ns electrons before the (n – 1)d electrons.

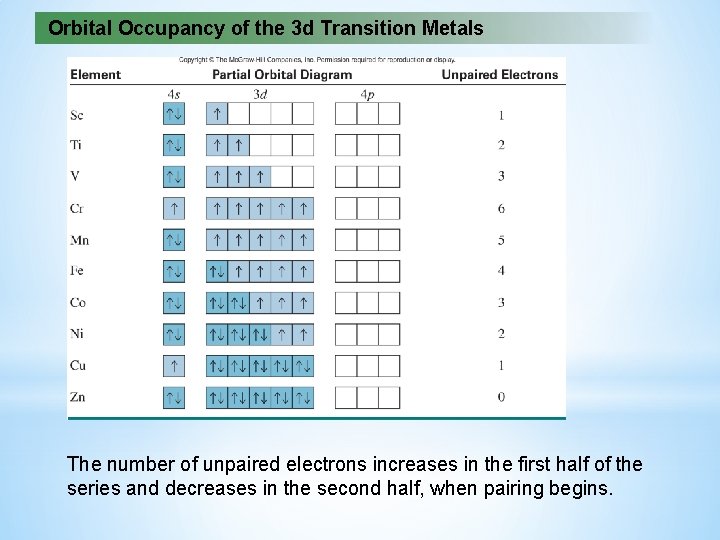
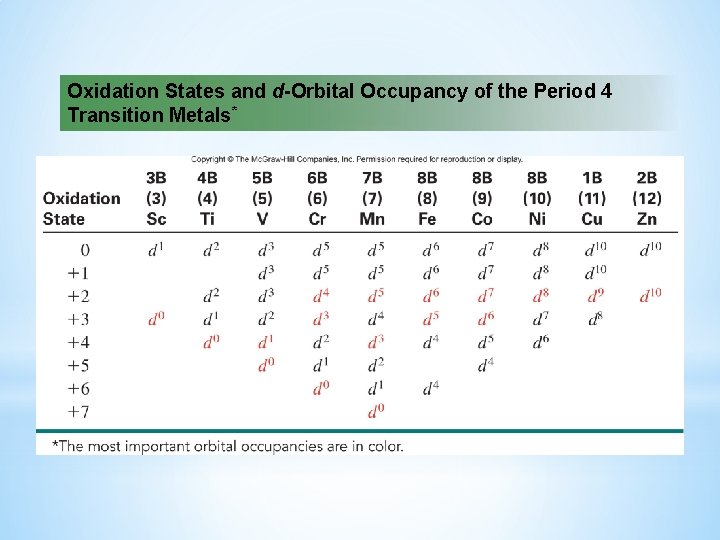
Orbital Occupancy of the 3 d Transition Metals The number of unpaired electrons increases in the first half of the series and decreases in the second half, when pairing begins.

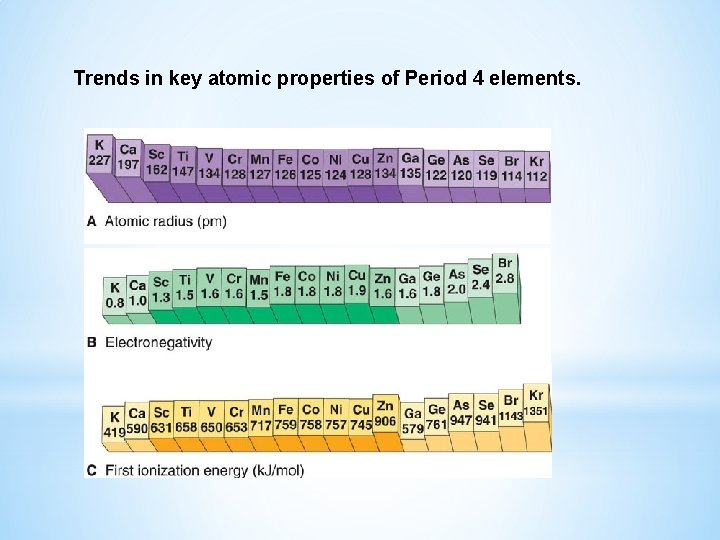
Trends in the Properties of Transition Metals Across a period the following trends are observed: Atomic size decreases at first, then remains relatively constant. - The d electrons fill inner orbitals, so they shield outer electrons very efficiently and the 4 s electrons are not pulled closer by the increasing nuclear charge. Electronegativity and ionization energies also increase relatively little across the transition metals of a particular period.

Trends in key atomic properties of Period 4 elements.

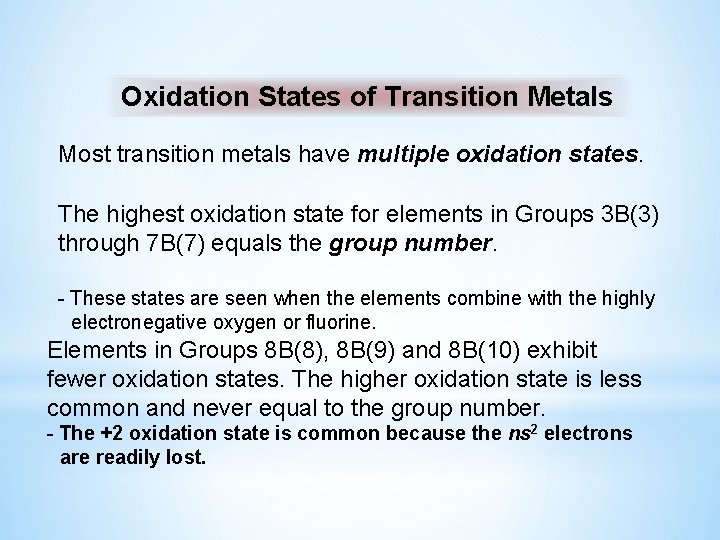
Oxidation States of Transition Metals Most transition metals have multiple oxidation states. The highest oxidation state for elements in Groups 3 B(3) through 7 B(7) equals the group number. - These states are seen when the elements combine with the highly electronegative oxygen or fluorine. Elements in Groups 8 B(8), 8 B(9) and 8 B(10) exhibit fewer oxidation states. The higher oxidation state is less common and never equal to the group number. - The +2 oxidation state is common because the ns 2 electrons are readily lost.

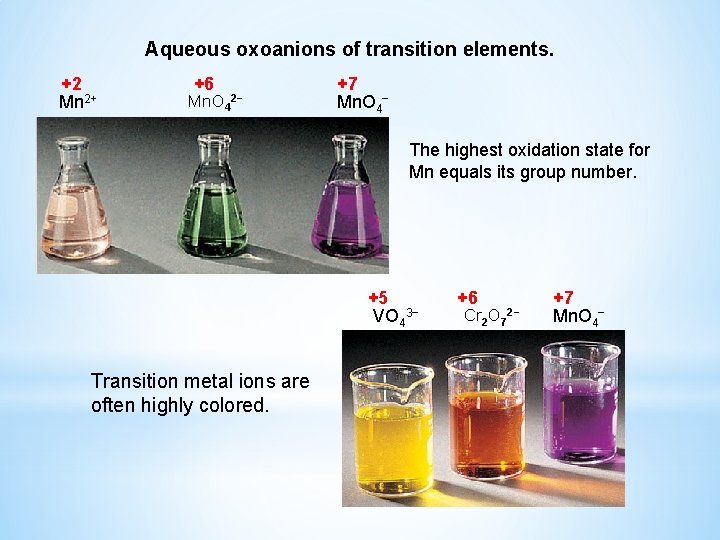
Aqueous oxoanions of transition elements. +2 Mn 2+ +6 Mn. O 4 2− +7 Mn. O 4− The highest oxidation state for Mn equals its group number. +5 VO 43− Transition metal ions are often highly colored. +6 Cr 2 O 7 2− +7 Mn. O 4−

Oxidation States and d-Orbital Occupancy of the Period 4 Transition Metals*

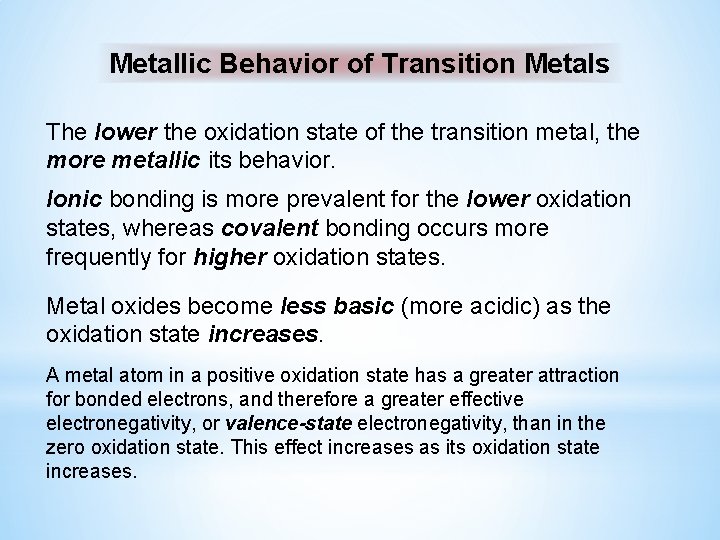
Metallic Behavior of Transition Metals The lower the oxidation state of the transition metal, the more metallic its behavior. Ionic bonding is more prevalent for the lower oxidation states, whereas covalent bonding occurs more frequently for higher oxidation states. Metal oxides become less basic (more acidic) as the oxidation state increases. A metal atom in a positive oxidation state has a greater attraction for bonded electrons, and therefore a greater effective electronegativity, or valence-state electronegativity, than in the zero oxidation state. This effect increases as its oxidation state increases.

Standard Electrode Potentials of 3 d- M 2+ Ions Half-Reaction E°(V) Ti 2+(aq) + 2 e− Ti(s) -1. 63 V 2+(aq) + 2 e− V(s) -1. 19 Cr 2+(aq) + 2 e− Cr(s) -0. 91 Mn 2+(aq) + 2 e− Mn(s) -1. 18 Fe 2+(aq) + 2 e− Fe(s) -0. 44 Co 2+(aq) + 2 e− Co(s) -0. 28 Ni 2+(aq) + 2 e− Ni(s) -0. 25 Cu 2+(aq) + 2 e− Cu(s) 0. 34 Zn 2+(aq) + 2 e− Zn(s) -0. 76 In general, reducing strength decreases across the series.

Color and Magnetic Behavior Most main-group ionic compounds are colorless and diamagnetic because the metal ion has no unpaired electrons. Many transition metal ionic compounds are highly colored and paramagnetic because the metal ion has one or more unpaired electrons. Transition metal ions with a d 0 or d 10 configuration are also colorless and diamagnetic.

Colors of representative compounds of the 3 dtransition metals. potassium nickel(II) nitrate zinc sulfate titanium(IV) oxide sodium chromate ferricyanide hexahydrate heptahydrate scandium oxide vanadyl sulfate manganese(II) dihydrate chloride tetrahydrate cobalt(II) chloride hexahydrate copper(II) sulfate pentahydrate

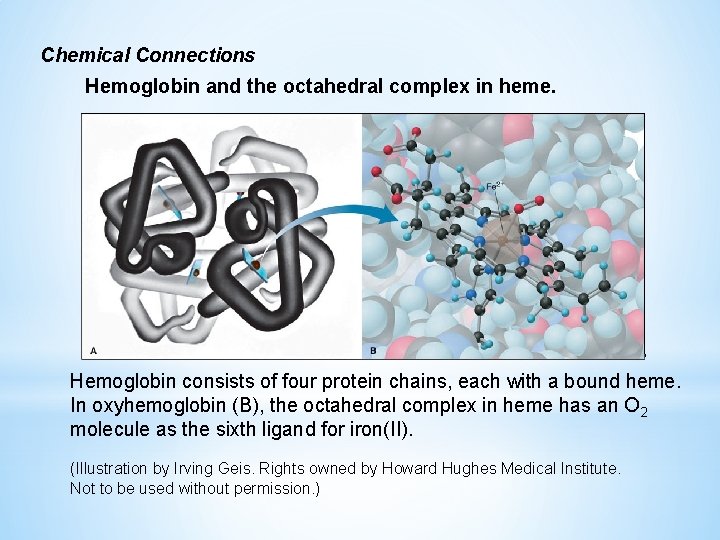
Chemical Connections Hemoglobin and the octahedral complex in heme. Hemoglobin consists of four protein chains, each with a bound heme. In oxyhemoglobin (B), the octahedral complex in heme has an O 2 molecule as the sixth ligand for iron(II). (Illustration by Irving Geis. Rights owned by Howard Hughes Medical Institute. Not to be used without permission. )

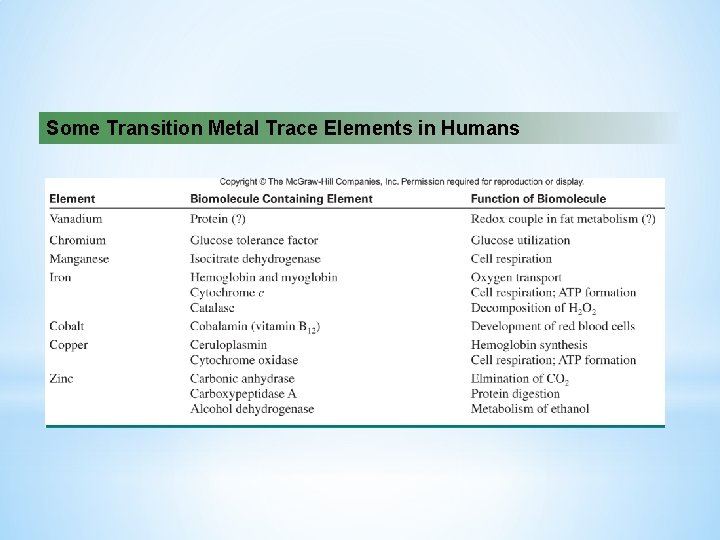
Some Transition Metal Trace Elements in Humans