3 4 Diffusion and Osmosis Somewhere on pg














- Slides: 30

3. 4 Diffusion and Osmosis Somewhere on pg. 28 If the cell membrane was rigid (solid or hard), as opposed to fluid (oil or liquid like), explain why the membrane would no longer be selectively permeable.

3. 4 Diffusion and Osmosis If the membrane was rigid: • The phospholipids would not be able to move from side to side or slide past one another • The phospholipids would be unable to move to allow material in or out!

3. 3 Cell Membrane Sponge: Set up Cornell Notes on pg. 31 Topic: 3. 4 Diffusion Essential Question: Explain what a concentration gradient is and what it means for a molecule to diffuse down its concentration gradient. 3. 4 Diffusion 2. 1 Atoms, Ions, and Molecules Explain what a concentration gradient is and what it means for a molecule to diffuse down its concentration gradient Key Concept: Materials move across membranes because of concentration differences.

3. 4 Diffusion and Osmosis 3. 3 Cell Membrane Standard 1. a Students know cells are enclosed within a semipermeable membranes that regulate their interaction with their surroundings.

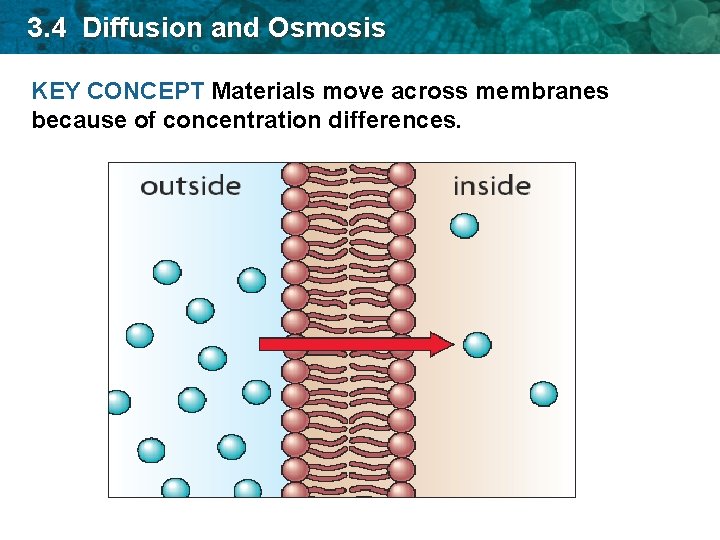
3. 4 Diffusion and Osmosis KEY CONCEPT Materials move across membranes because of concentration differences.

3. 4 Diffusion and Osmosis Solution: A homogenous mixture of two or more substances, which may be solids, liquids, or gases, or the combination of these. Ex: Sugar water Solute: Substance dissolved in another substance Ex: Sugar Solvent: Substance doing the dissolving (usually the substance present in the greatest amount) Ex: Water (THE BEST SOLVENT)

Which one is the Solute? Solvent? Solution? Milk Solvent Air Solution Chocolate Milk Solution Chocolate Syrup Solute Nitrogen (78%) Oxygen, argon, CO 2 (22%) Solvent Solute

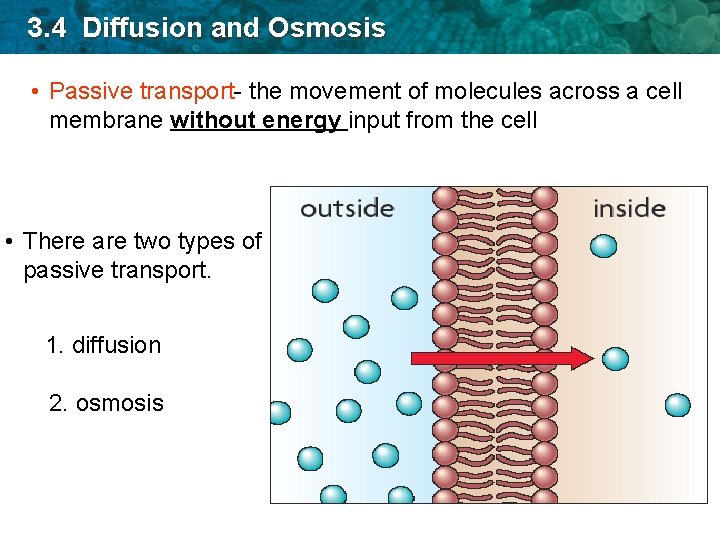
3. 4 Diffusion and Osmosis • Passive transport- the movement of molecules across a cell membrane without energy input from the cell • There are two types of passive transport. 1. diffusion 2. osmosis

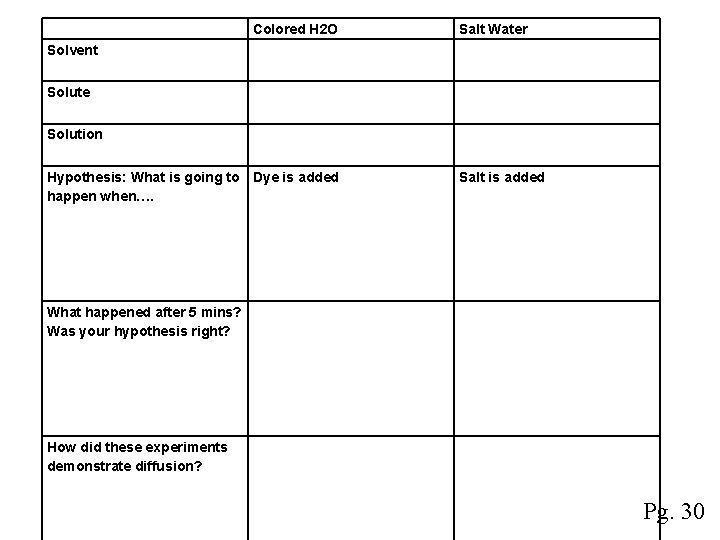
Diffusion Demonstration Materials: 2 Cups, Water, Dye, Salt, Graph 1) 2) 3) 4) Identify the solvent, solute, and solution for each demonstration Hypothesize what is going to happen when you drop the food coloring in the water. Salt in the water? Did your hypothesis match the results? Explain how these experiments demonstrated diffusion

Colored H 2 O Salt Water Solvent Solute Solution Hypothesis: What is going to Dye is added happen when…. What happened after 5 mins? Was your hypothesis right? How did these experiments demonstrate diffusion? Salt is added Pg. 30

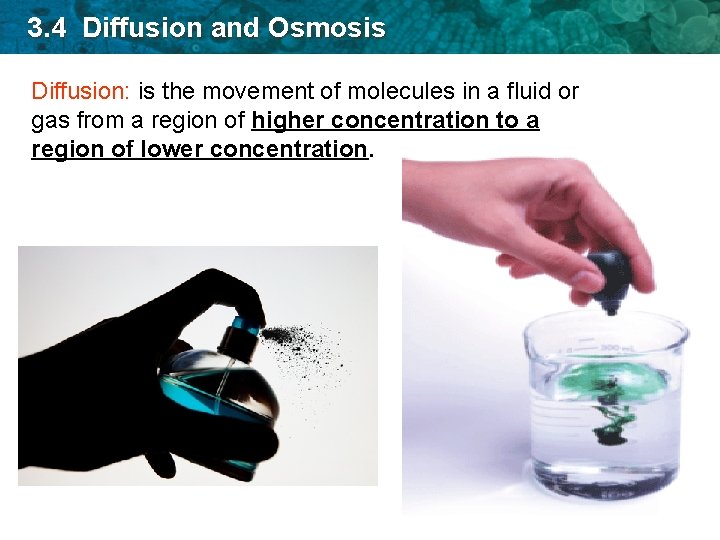
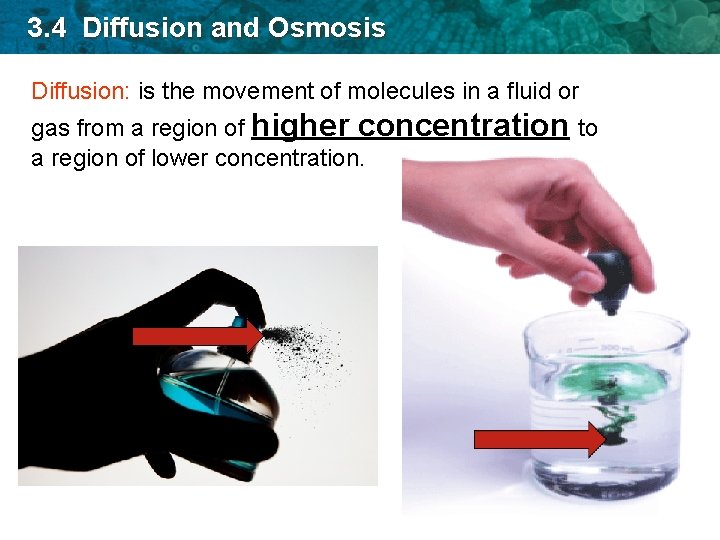
3. 4 Diffusion and Osmosis Diffusion: is the movement of molecules in a fluid or gas from a region of higher concentration to a region of lower concentration.

3. 4 Diffusion and Osmosis Diffusion: is the movement of molecules in a fluid or gas from a region of higher concentration to a region of lower concentration.

3. 4 Diffusion and Osmosis Diffusion: is the movement of molecules in a fluid or gas from a region of higher concentration to a region of lower concentration.

Air Freshener Demo.

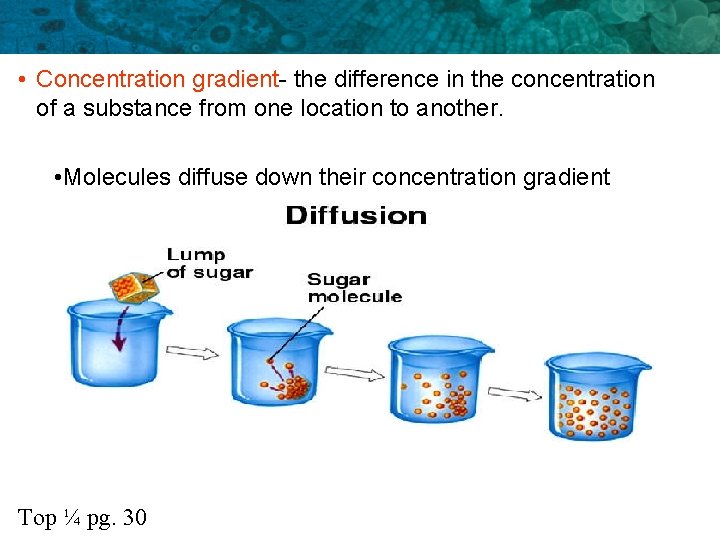
• Concentration gradient- the difference in the concentration of a substance from one location to another. • Molecules diffuse down their concentration gradient Top ¼ pg. 30

3. 3 Cell Membrane Sponge: Set up Cornell Notes on pg. 33 Topic: 3. 4 Osmosis Essential Question: A cell is bathed in fluid. However, you notice that water is flowing out of the cell. In what kind of solution is this cell immersed? 3. 4 Osmosis 2. 1 Atoms, Ions, and Molecules A cell is bathed in fluid. However, you notice that water is flowing out of the cell. In what kind of solution is this cell immersed?

3. 3 Cell Membrane Isotonic Solution Pg. 32 Hypertonic Solution Hypotonic Solution

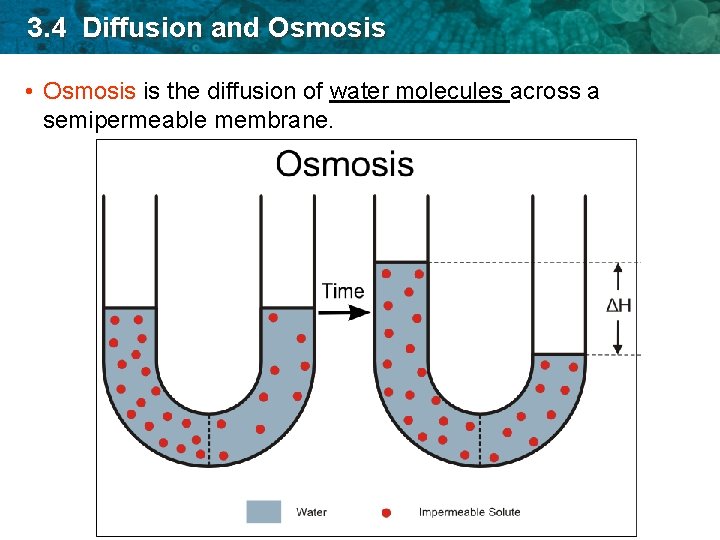
3. 4 Diffusion and Osmosis • Osmosis is the diffusion of water molecules across a semipermeable membrane.

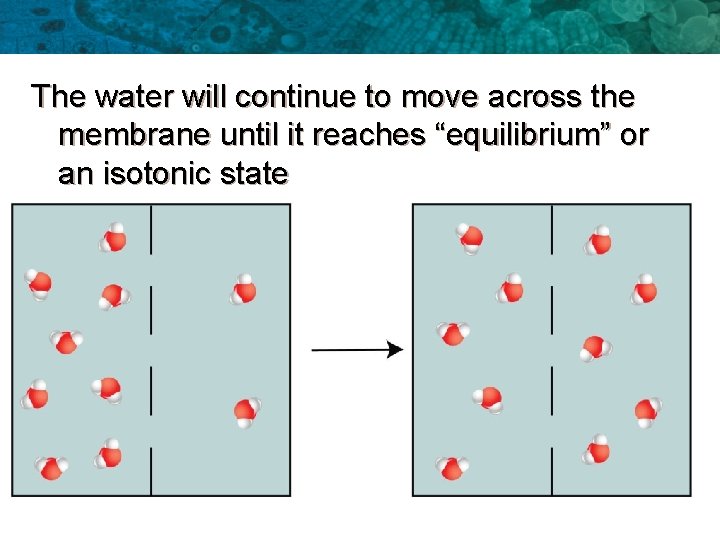
The water will continue to move across the membrane until it reaches “equilibrium” or an isotonic state

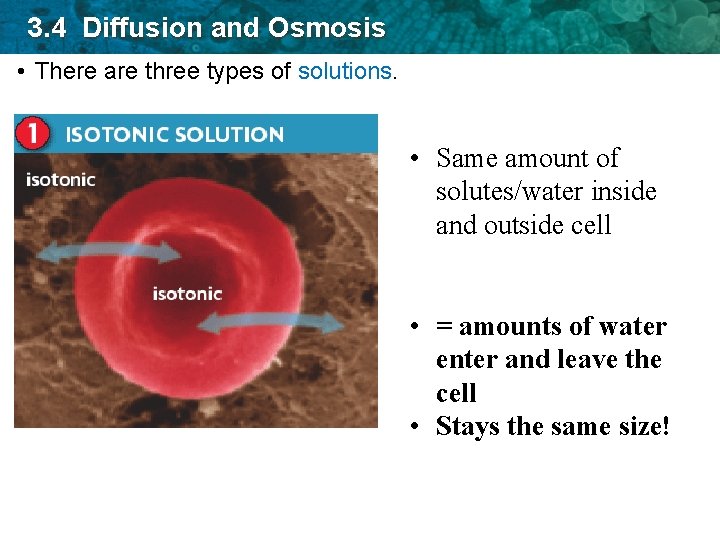
3. 4 Diffusion and Osmosis • There are three types of solutions. • Same amount of solutes/water inside and outside cell • = amounts of water enter and leave the cell • Stays the same size!

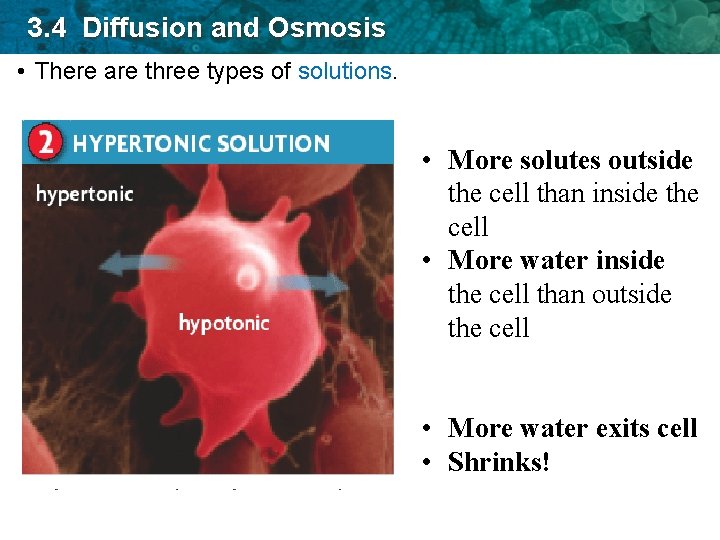
3. 4 Diffusion and Osmosis • There are three types of solutions. • More solutes outside the cell than inside the cell • More water inside the cell than outside the cell • More water exits cell • Shrinks!

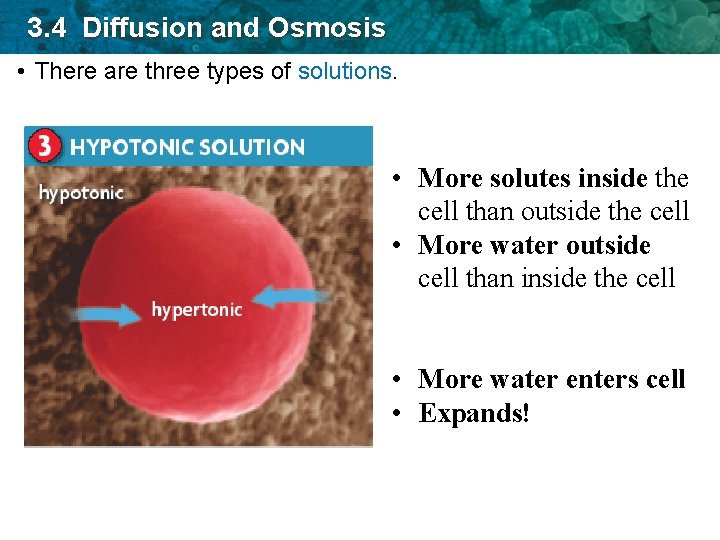
3. 4 Diffusion and Osmosis • There are three types of solutions. • More solutes inside the cell than outside the cell • More water outside cell than inside the cell • More water enters cell • Expands!

Osmosis Videos Osmosis vs. Diffusion • Osmosis Computer Graphics • Osmosis Demo Video • Osmosis with a U-tube •

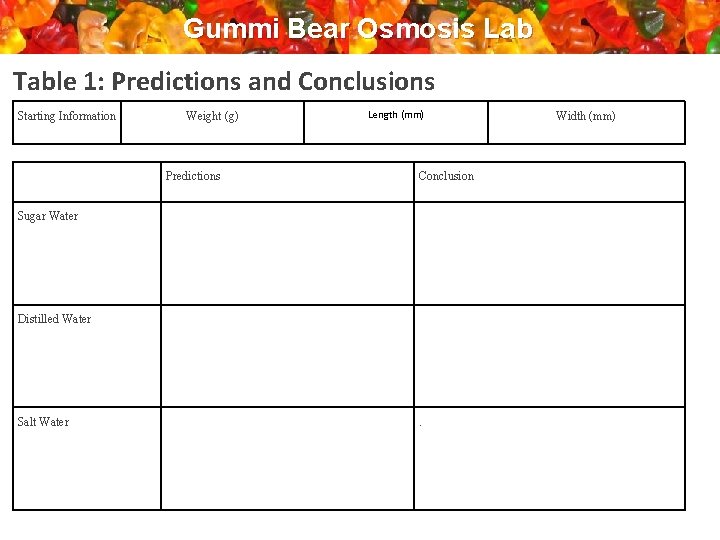
Gummi Bear Osmosis Lab Table 1: Predictions and Conclusions Starting Information Weight (g) Length (mm) Predictions Conclusion Sugar Water Distilled Water Salt Water . Width (mm)

Osmosis Lab Materials: 1 cup Water Sugar 1 Gummi Bear Scale (g) Ruler (mm) String (for width) Salt 1) Label each cup: First/Last Name Period #

Day One: Gathering Data 1) Weigh your Gummi Bear and record its weight (g) 2) Measure your Gummi’s length and width (mm) 3) Place the Gummi in a 25/75 sugar/water solution 4) Fill in your table with your hypothesis

Day Two: Gathering Data 1) 2) 3) 4) Dry your Gummi bear off carefully Weigh your Gummi Bear and record its weight (g) Measure your Gummi’s length and width (mm) Was your hypothesis correct? What happened to your Gummi? Compare this data with your group data, and record on Lab report tables. Rinse out your cup and rinse off your gummi (quick and carefully) 6) Place the Gummi in a 100% distilled water 5) 7) Fill in your table with your new hypothesis

Day Three: Gathering Data 1) 2) 3) 4) Dry your Gummi bear off carefully Weigh your Gummi Bear and record its weight (g) Measure your Gummi’s length and width (mm) Was your hypothesis correct? What happened to your Gummi? Compare this data with your group data, and record on Lab report tables. Rinse out your cup and rinse off your gummi (quick and carefully) 6) Place the Gummi in a 25/75 solution of salt and water 7) Fill in your table with your new hypothesis 5)

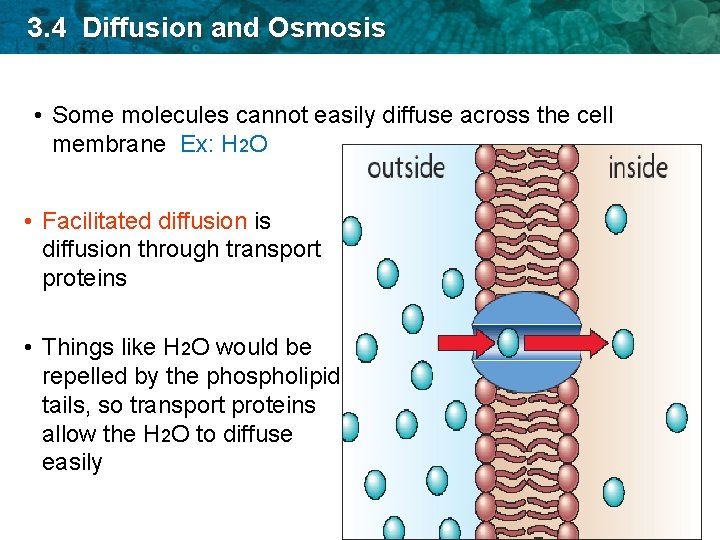
3. 4 Diffusion and Osmosis • Some molecules cannot easily diffuse across the cell membrane Ex: H 2 O • Facilitated diffusion is diffusion through transport proteins • Things like H 2 O would be repelled by the phospholipid tails, so transport proteins allow the H 2 O to diffuse easily

Pg. 32 Diffusion and Osmosis Video Notes Osmosis in the kitchen • Osmosis with an Egg • Desalination- Reverse Osmosis •