3 2 Synthesis Decomposition and Combustion Reactions Chemical

3. 2 Synthesis, Decomposition and Combustion Reactions
Chemical Reactions u Chemical reactions can be grouped into five categories: 1. 2. 3. 4. 5. Synthesis/Combination Decomposition Combustion Single displacement Double displacement

Synthesis and Decomposition Reactions
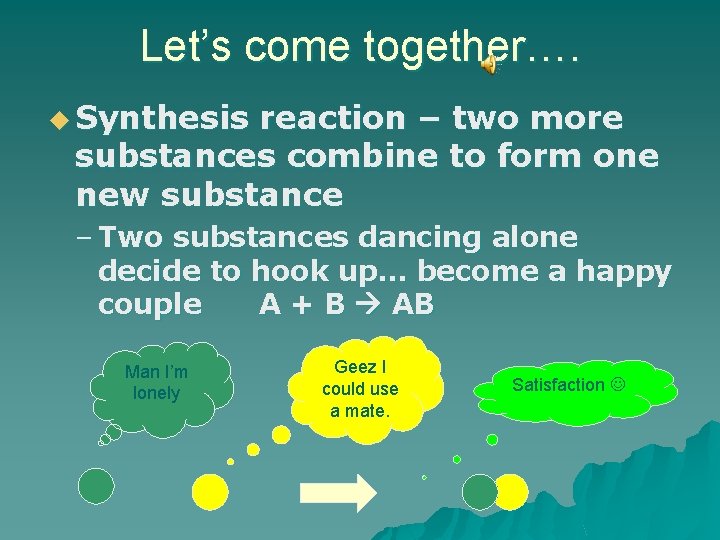
Let’s come together…. u Synthesis reaction – two more substances combine to form one new substance – Two substances dancing alone decide to hook up… become a happy couple A + B AB Man I’m lonely Geez I could use a mate. Satisfaction

Synthesis Reaction u Usually exothermic (energy is produced) u Can occur naturally or by an initial application of energy (heat, flame, UV light, use of catalyst) Let’s come together….
Synthesis Reaction Man I’m lonely Geez I could use a mate. Satisfaction Let’s come together….

Practice u Show the synthesis of solid aluminum and oxygen gas. DIATOMIC – What are the reactants? u. Al + O 2 ALERT! – What are the products? u. It’s a synthesis reaction, so we know Al and O are going to make a compound…. u. If the products aren’t in the question you need to KRISS KROSS to figure it out! Al (s) + O 2 (g) 3+ 2 Al Al. O O 3 2 O
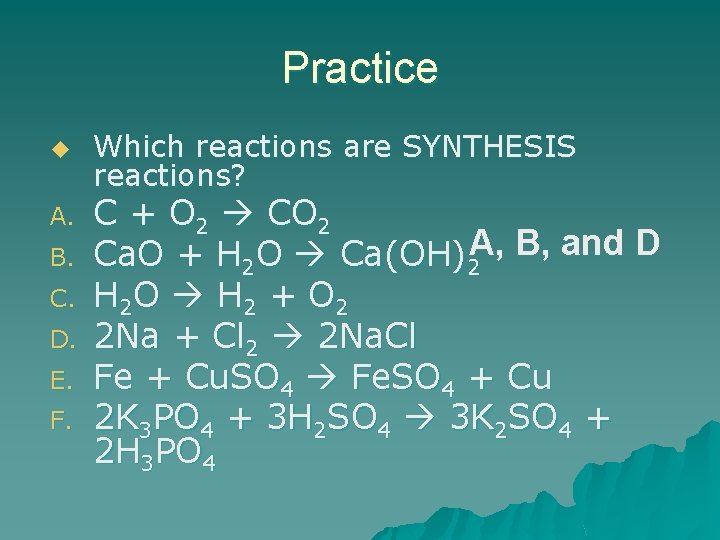
Practice u A. B. C. D. E. F. Which reactions are SYNTHESIS reactions? C + O 2 CO 2 Ca. O + H 2 O Ca(OH)2 A, B, and D H 2 O H 2 + O 2 2 Na + Cl 2 2 Na. Cl Fe + Cu. SO 4 Fe. SO 4 + Cu 2 K 3 PO 4 + 3 H 2 SO 4 3 K 2 SO 4 + 2 H 3 PO 4
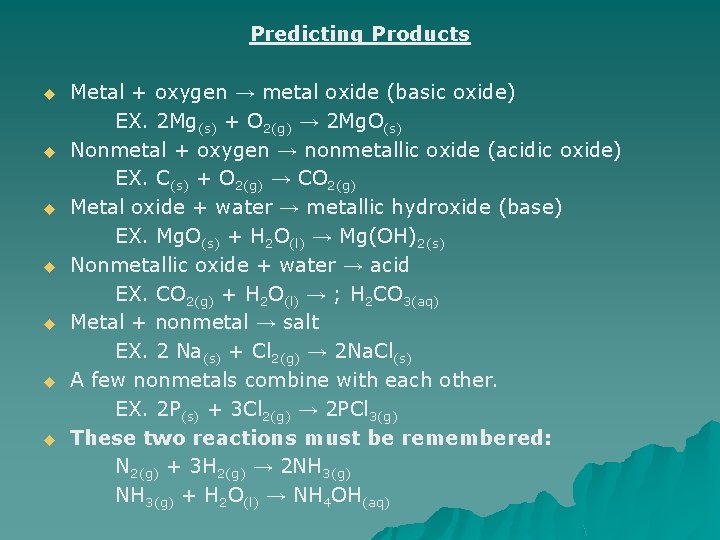
Predicting Products u u u u Metal + oxygen → metal oxide (basic oxide) EX. 2 Mg(s) + O 2(g) → 2 Mg. O(s) Nonmetal + oxygen → nonmetallic oxide (acidic oxide) EX. C(s) + O 2(g) → CO 2(g) Metal oxide + water → metallic hydroxide (base) EX. Mg. O(s) + H 2 O(l) → Mg(OH)2(s) Nonmetallic oxide + water → acid EX. CO 2(g) + H 2 O(l) → ; H 2 CO 3(aq) Metal + nonmetal → salt EX. 2 Na(s) + Cl 2(g) → 2 Na. Cl(s) A few nonmetals combine with each other. EX. 2 P(s) + 3 Cl 2(g) → 2 PCl 3(g) These two reactions must be remembered: N 2(g) + 3 H 2(g) → 2 NH 3(g) + H 2 O(l) → NH 4 OH(aq)

I don’t think this is working out… u Decomposition reaction – one substance breaks into multiple substances – A couple decides…well it is time to break up AB A + B I don’t think this is right for us… I want to see other people Satisfaction!
Decomposition Reaction u Usually endothermic (requires energy) u Can require energy in the form of heat, electricity, catalyst, UV light u *some decomposition rxns occur at room temperature
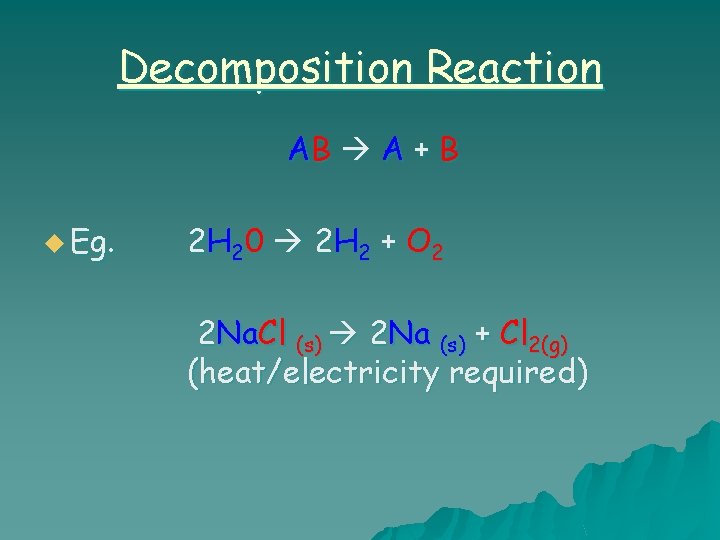
Decomposition Reaction AB A + B u Eg. 2 H 2 0 2 H 2 + O 2 2 Na. Cl (s) 2 Na (s) + Cl 2(g) (heat/electricity required)
Decomposition Reaction I don’t think this is right for us… Satisfaction! I want to see other people Satisfaction!

Practice u Show the decomposition water after electricity has passed through it. – What are the reactants? DIATOMIC ALERT! – What are the products? u. Water! H 2 O u. It’s a decomposition reaction, so we know the elements in water are going to break apart u. The question will specify if any compounds are formed H 2 O H 2++OO 2 Don’t forget to balance!

More Practice! u A. B. C. D. E. F. Which reactions are DECOMPOSITION reactions? C + O 2 CO 2 Ca. O + H 2 O Ca(OH)2 C, D and F H 2 O H 2 + O 2 H 2 SO 4 H 2 + S + 2 O 2 Fe + Cu. SO 4 Fe. SO 4 + Cu K 3 PO 4 K 3 P + 2 O 2
u u u Predicting Products Metallic carbonates, when heated, form metallic oxides and CO 2(g). EX. Ca. CO 3(s) → Ca. O(s) + CO 2(g) Most metallic hydroxides, when heated, decompose into metallic oxides and water. EX. Ca(OH)2(s) → Ca. O(s) + H 2 O(g) Metallic chlorates, when heated, decompose into metallic chlorides and oxygen. EX. 2 KCl. O 3(s) → 2 KCl(s) + 3 O 2(g) Some acids, when heated, decompose into nonmetallic oxides and water. EX. H 2 SO 4 → H 2 O(l) + SO 3(g) Some oxides, when heated, decompose. EX. 2 Hg. O(s) → 2 Hg(l) + O 2(g) Some decomposition reactions are produced by electricity. EX. 2 H 2 O(l) → 2 H 2(g) + O 2(g) EX. 2 Na. Cl(l) → 2 Na(s) + Cl 2(g)
Combustion Reactions When a compound or element is reacted with O 2 (g) to form the most common oxides that make up that compound. -These reactions usually accompany heat and light. Complete combustion – products H 2 O and CO 2 Incomplete Combustion - products H 2 O and CO
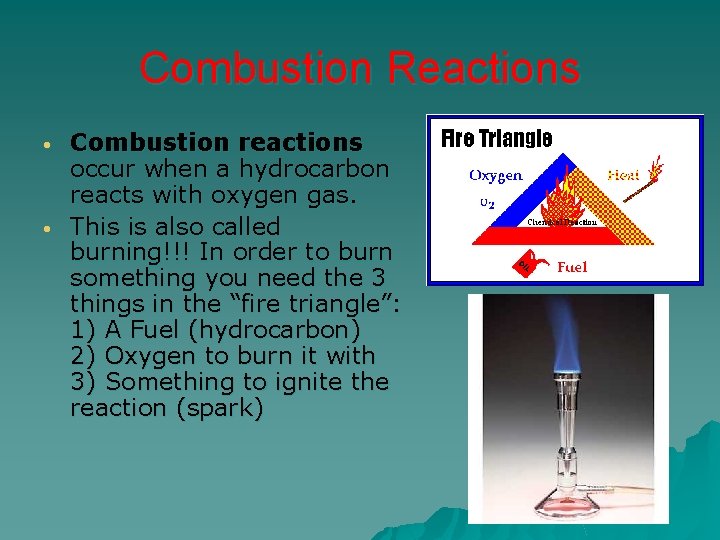
Combustion Reactions • • Combustion reactions occur when a hydrocarbon reacts with oxygen gas. This is also called burning!!! In order to burn something you need the 3 things in the “fire triangle”: 1) A Fuel (hydrocarbon) 2) Oxygen to burn it with 3) Something to ignite the reaction (spark)

Combustion Reactions • • • In general: Cx. Hy + O 2 CO 2 + H 2 O Products in combustion are ALWAYS carbon dioxide and water. (although incomplete burning does cause some by -products like carbon monoxide) Combustion is used to heat homes and run automobiles (octane, as in gasoline, is C 8 H 18)

Combustion Reactions Edgar Allen Poe’s drooping eyes and mouth are potential signs of CO poisoning.
- Slides: 20