3 2 Composition and Structure of Metallic Materials







- Slides: 38




3 -2 金属材料的结构和组成 Composition and Structure of Metallic Materials • What is the relationship between crystal unit cells and electronic structures of metal atoms • How to calculate the crystal structure parameters and densities of metals • What is metal alloy and its main structural types • What is the iron-iron carbide phase diagram

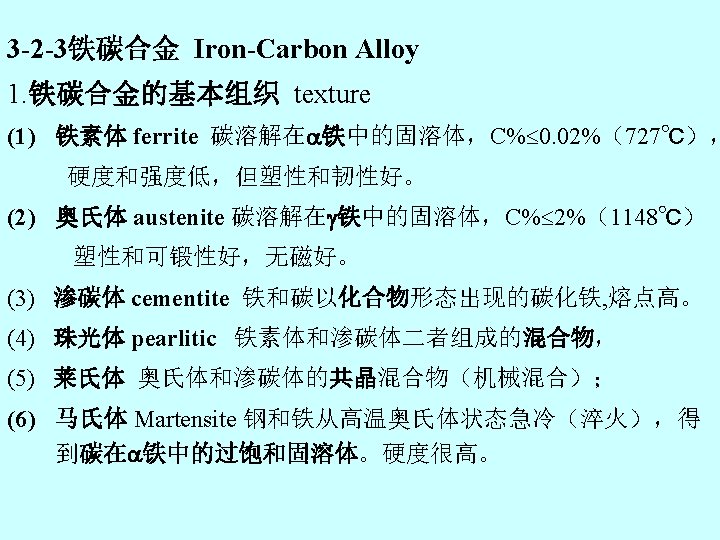
• crystal unit cells and electronic structures of metal atoms • Calculation of the crystal structure parameters and densities of metals, • metallic alloy and its main structural types: Solid solution, intermetallic compound, mechanical blends • Iron-Carbon Alloy Texture Ferrite, austenite, cementite , pearlitic • Iron-iron carbide phase diagram : development of microstructures in alloy, eutectoid steel , eutectic steel • Steel and Cast Iron • Copper and its Alloys • Noncrystalline metallic alloy , • Recrystalliny of Metals

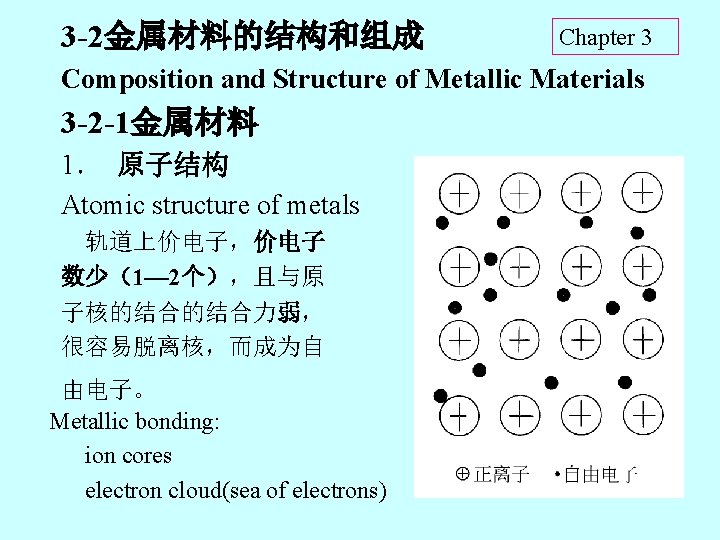
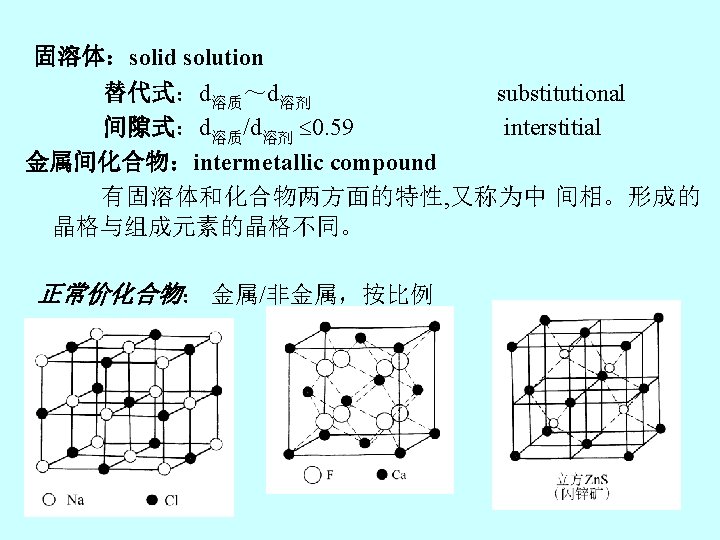
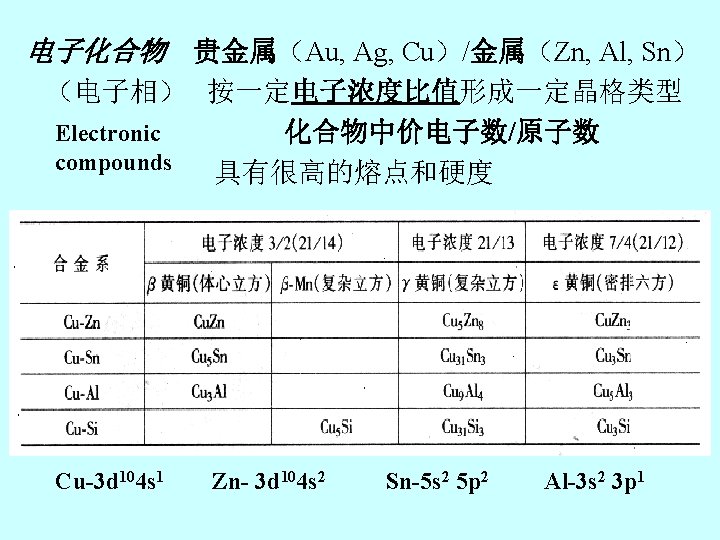
3 -2金属材料的结构和组成 Chapter 3 Composition and Structure of Metallic Materials 3 -2 -1金属材料 1. 原子结构 Atomic structure of metals 轨道上价电子,价电子 数少(1— 2个),且与原 子核的结合的结合力弱, 很容易脱离核,而成为自 由电子。 Metallic bonding: ion cores electron cloud(sea of electrons)


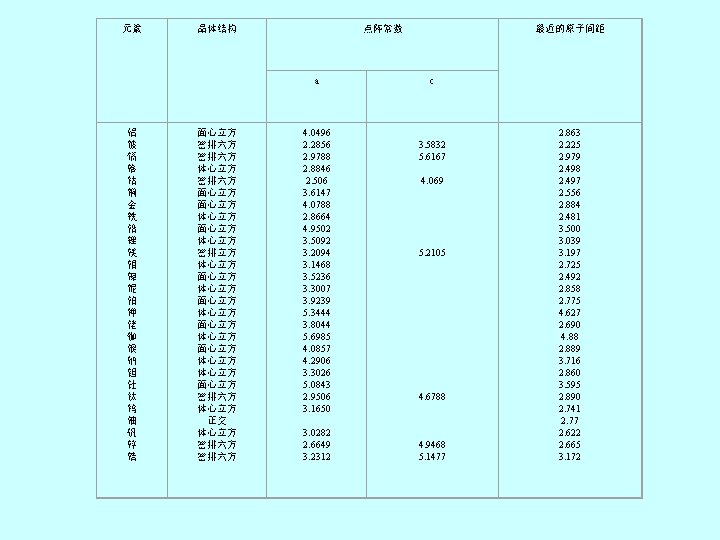
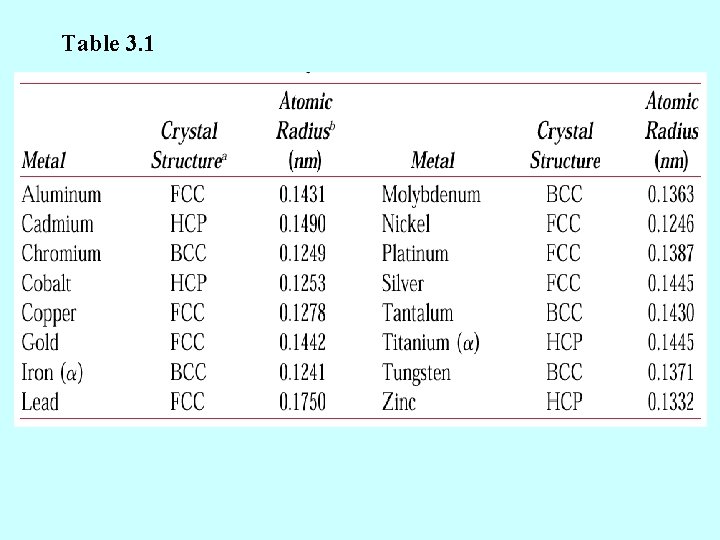
元素 铝 铍 镉 铬 钴 铜 金 铁 铅 锂 镁 钼 镍 铌 铂 钾 铑 铷 银 钠 钽 钍 钛 钨 铀 钒 锌 锆 点阵常数 晶体结构 面心立方 密排六方 体心立方 密排六方 面心立方 体心立方 密排立方 体心立方 面心立方 体心立方 面心立方 密排六方 体心立方 正交 体心立方 密排六方 最近的原子间距 a c 4. 0496 2. 2856 2. 9788 2. 8846 2. 506 3. 6147 4. 0788 2. 8664 4. 9502 3. 5092 3. 2094 3. 1468 3. 5236 3. 3007 3. 9239 5. 3444 3. 8044 5. 6985 4. 0857 4. 2906 3. 3026 5. 0843 2. 9506 3. 1650 3. 0282 2. 6649 3. 2312 3. 5832 5. 6167 4. 069 5. 2105 4. 6788 4. 9468 5. 1477 2. 863 2. 225 2. 979 2. 498 2. 497 2. 556 2. 884 2. 481 3. 500 3. 039 3. 197 2. 725 2. 492 2. 858 2. 775 4. 627 2. 690 4. 88 2. 889 3. 716 2. 860 3. 595 2. 890 2. 741 2. 77 2. 622 2. 665 3. 172

Table 3. 1

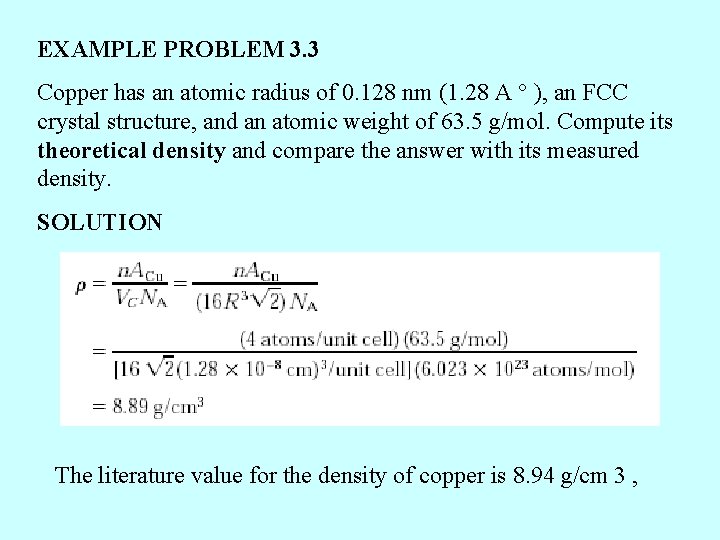
EXAMPLE PROBLEM 3. 3 Copper has an atomic radius of 0. 128 nm (1. 28 A ° ), an FCC crystal structure, and an atomic weight of 63. 5 g/mol. Compute its theoretical density and compare the answer with its measured density. SOLUTION The literature value for the density of copper is 8. 94 g/cm 3 ,

3. 4 Show for the body-centered cubic crystal Calculate the radius of a vanadium atom, given that V has a BCC crystal structure, a density of 5. 96 g/cm 3, and an atomic weight of 50. 9 g/mol.






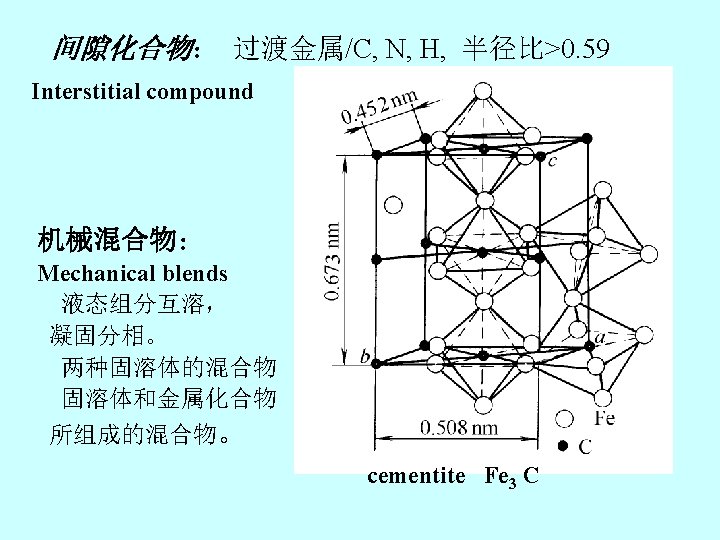
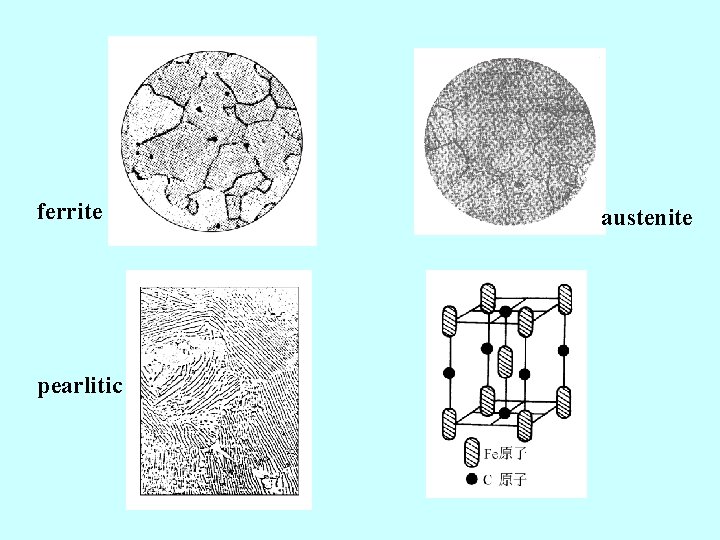
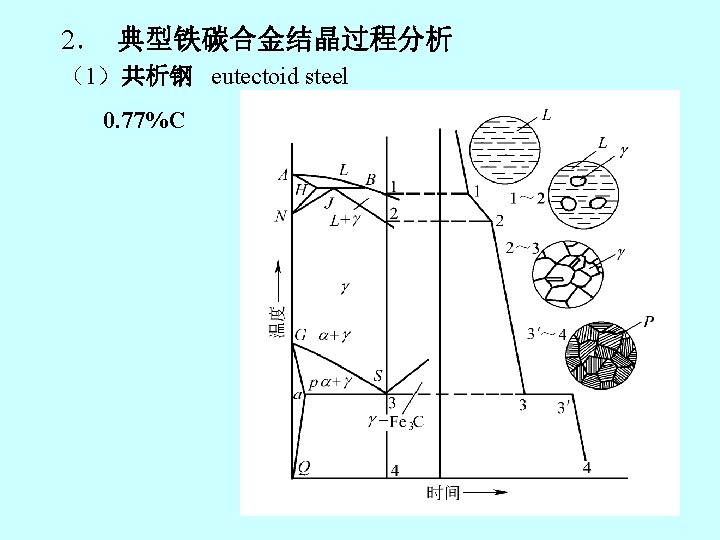
ferrite pearlitic austenite

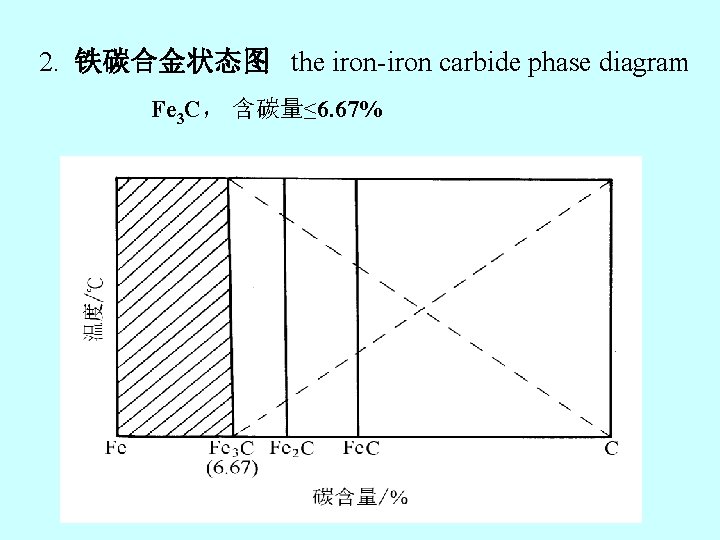
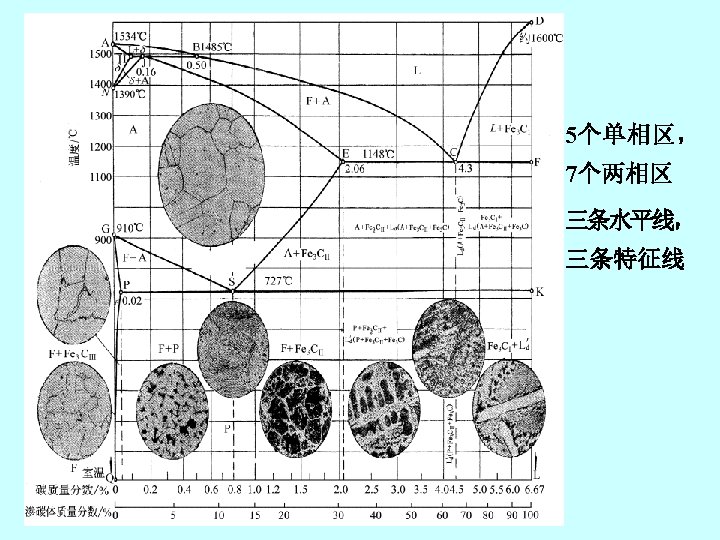
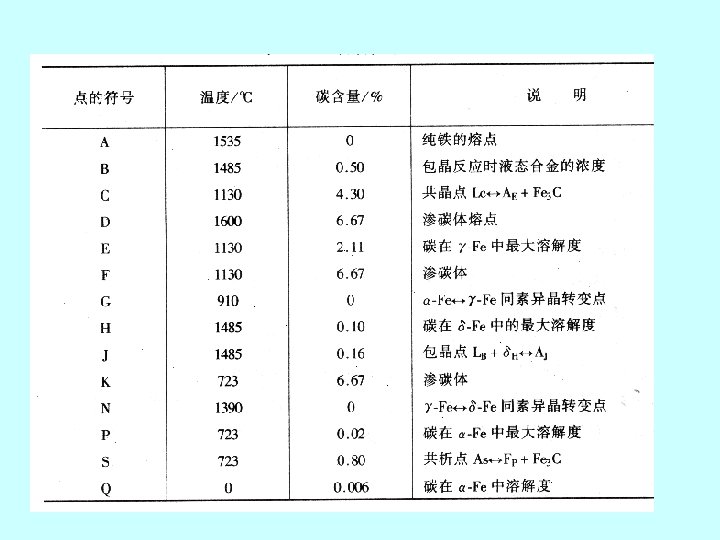
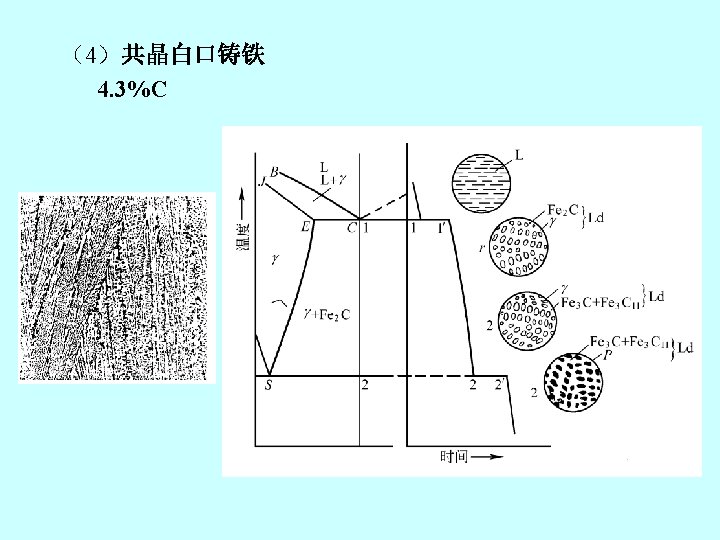
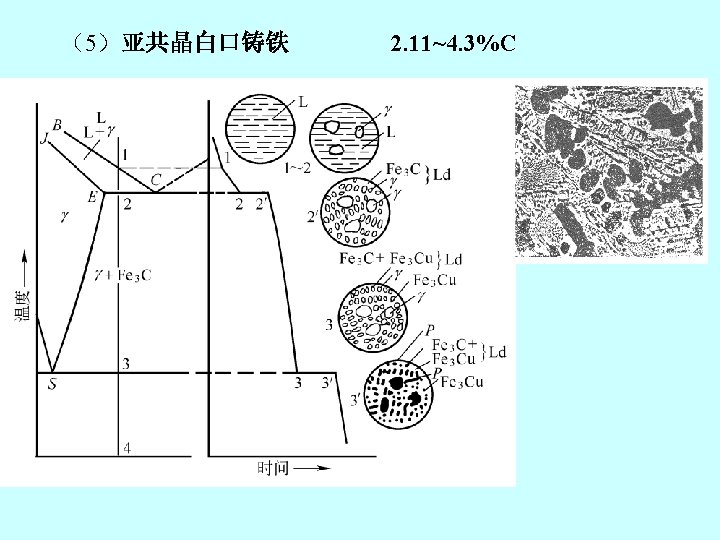
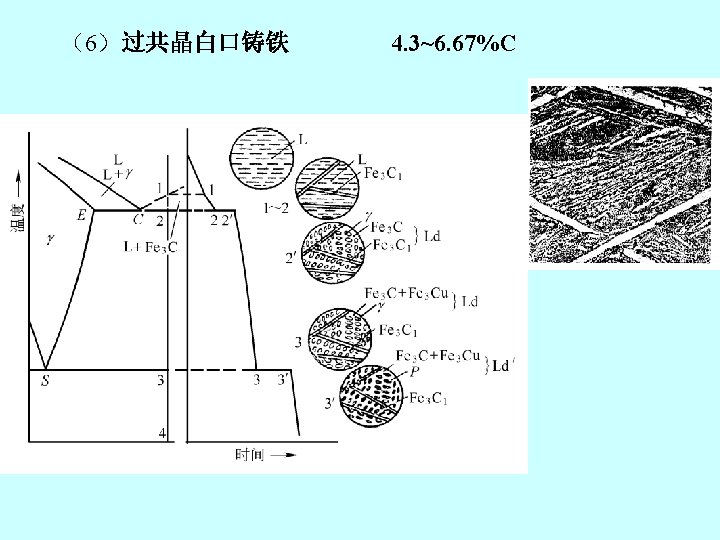
2. 铁碳合金状态图 the iron-iron carbide phase diagram Fe 3 C, 含碳量≤ 6. 67%




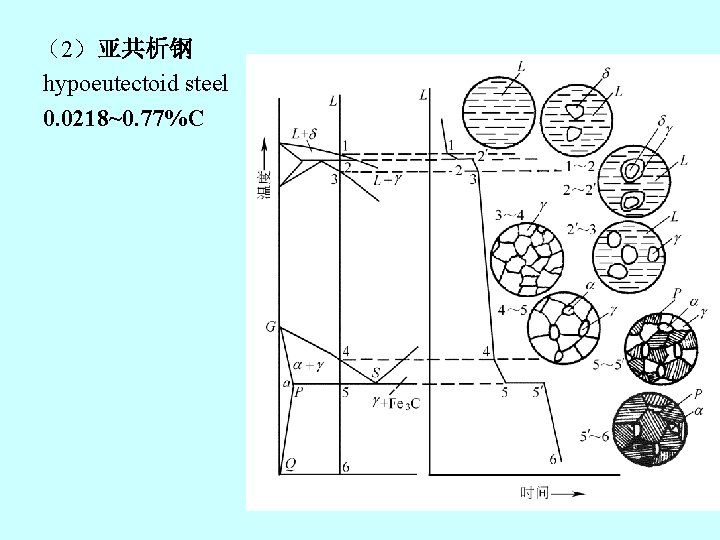
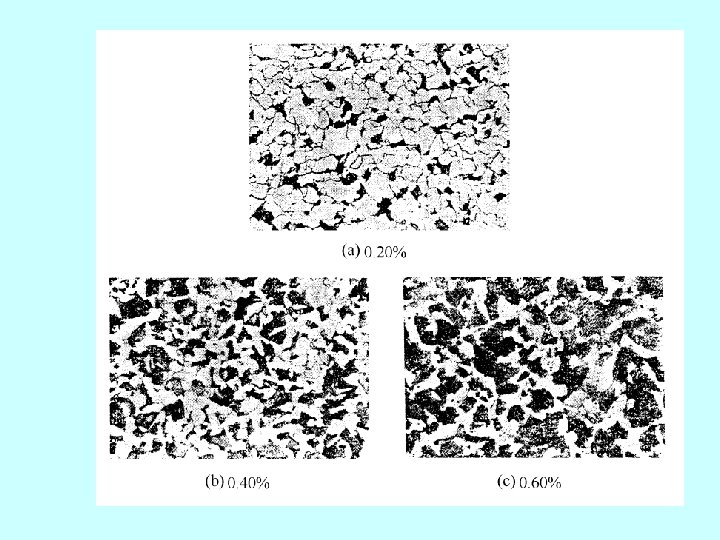
(2)亚共析钢 hypoeutectoid steel 0. 0218~0. 77%C


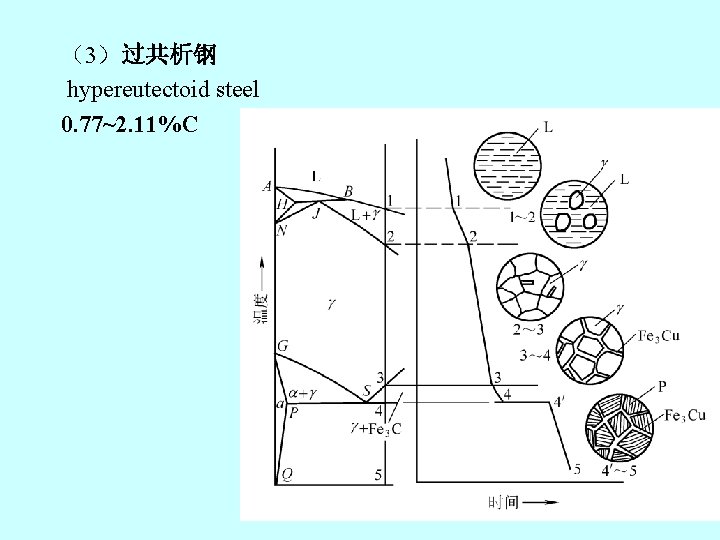
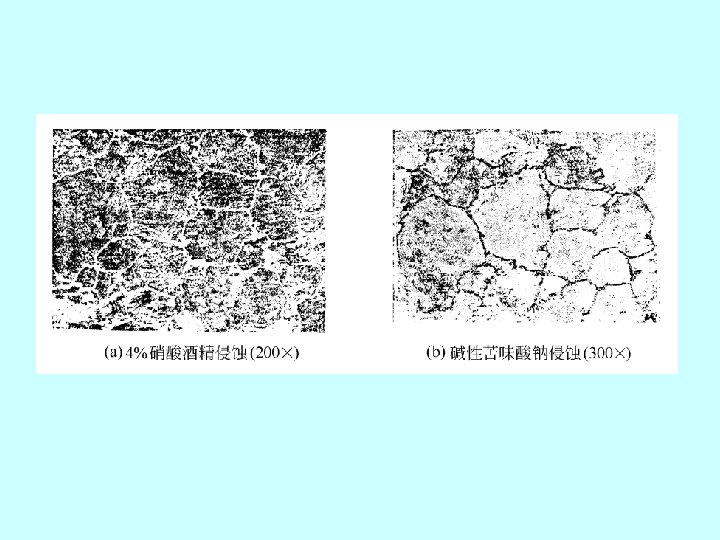
(3)过共析钢 hypereutectoid steel 0. 77~2. 11%C





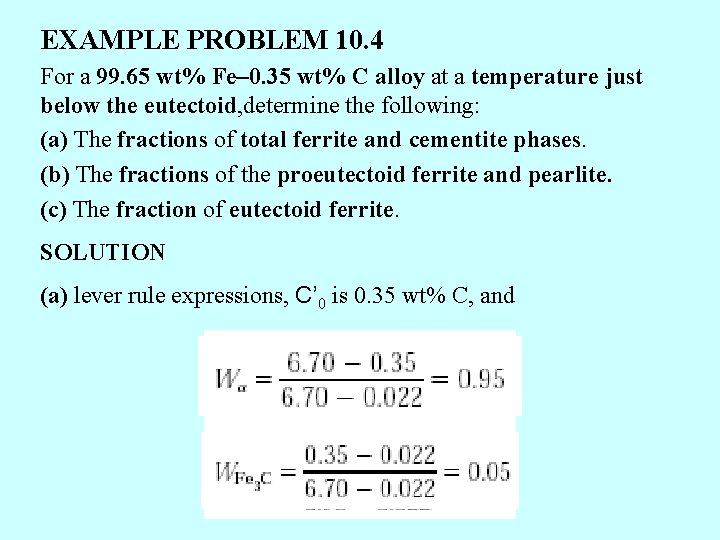
EXAMPLE PROBLEM 10. 4 For a 99. 65 wt% Fe– 0. 35 wt% C alloy at a temperature just below the eutectoid, determine the following: (a) The fractions of total ferrite and cementite phases. (b) The fractions of the proeutectoid ferrite and pearlite. (c) The fraction of eutectoid ferrite. SOLUTION (a) lever rule expressions, C’ 0 is 0. 35 wt% C, and

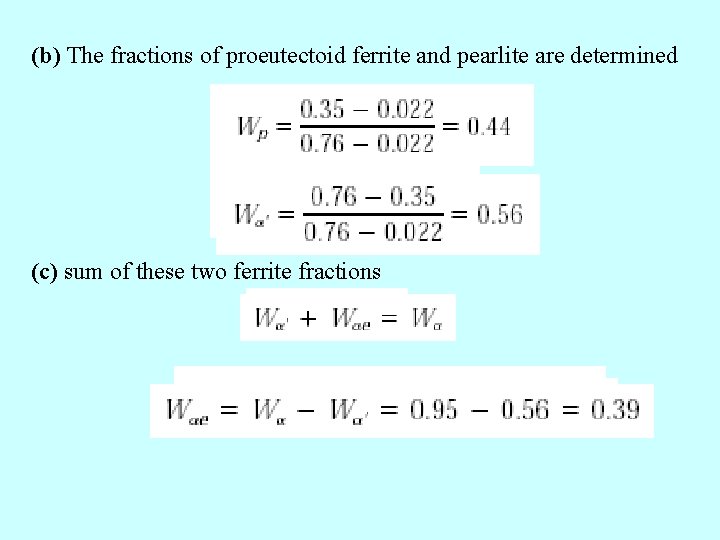
(b) The fractions of proeutectoid ferrite and pearlite are determined (c) sum of these two ferrite fractions







