3 2 Acid and Bases Acids and their
3. 2 Acid and Bases
Acids and their properties • An acid is any compound that increases the number of hydronium ions – Hydronium forms when and H+ separates from an acid and bonds with water = H 3 O+
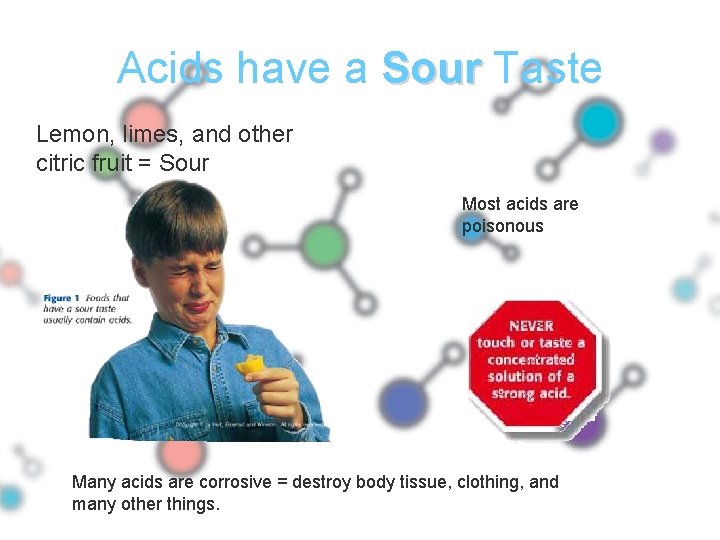
Acids have a Sour Taste Lemon, limes, and other citric fruit = Sour Most acids are poisonous Many acids are corrosive = destroy body tissue, clothing, and many other things.
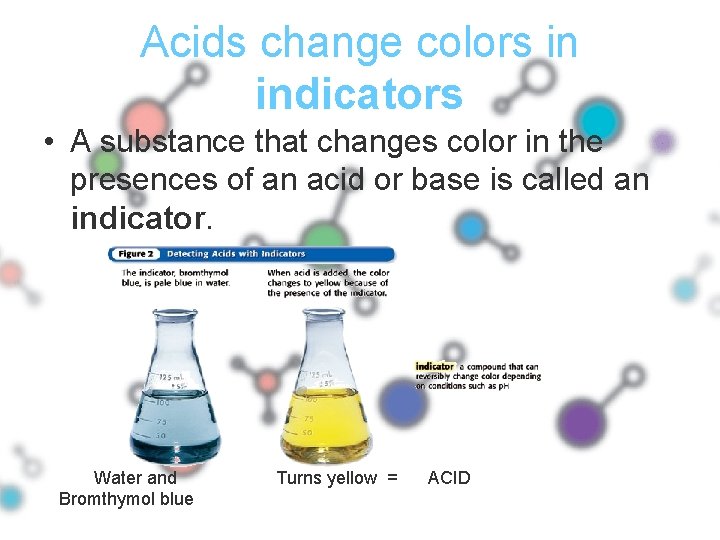
Acids change colors in indicators • A substance that changes color in the presences of an acid or base is called an indicator. Water and Bromthymol blue Turns yellow = ACID
Acids react with metals • Acids and metals = H+ gas • 2 HCl + Zn H 2 + Zn. Cl 2 (What type of reaction did you learn this was? AB + C A + CB)
Acids conduct electric current • Acids in water produce ions • These ions allow the solution to conduct electricity. • Like in this car battery.
Uses of Acids • Sulfuric Acid – Help make paper – Paint – Detergents – fertilizers • Hydrochloric acid – Swimming pool sanitizer – In your stomach— digests food • Hydrofluoric Acid • Etch glass

Uses of Acids • Citric Acid & Ascorbic Acid (vitamin C) – Orange Juice • Carbonic acid phosphoric acid • Help give flavor to soft drinks
Bases and their properties • A base is any compound that increases the number of hydroxide ions (OH-) when dissolved in water. • Example: Na. OH Na+ + OH- in water

Bases • Bases have a bitter flavor • Bases have a slippery feel – Example: • Soap • Be careful, many bases are corrosive!
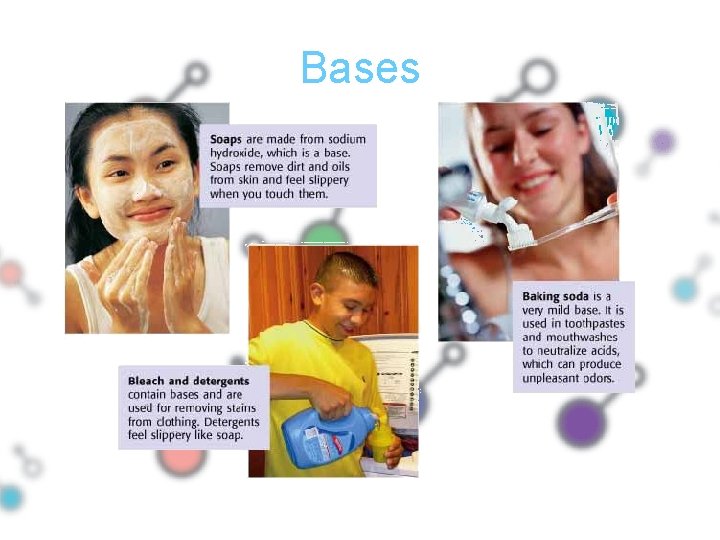
Bases
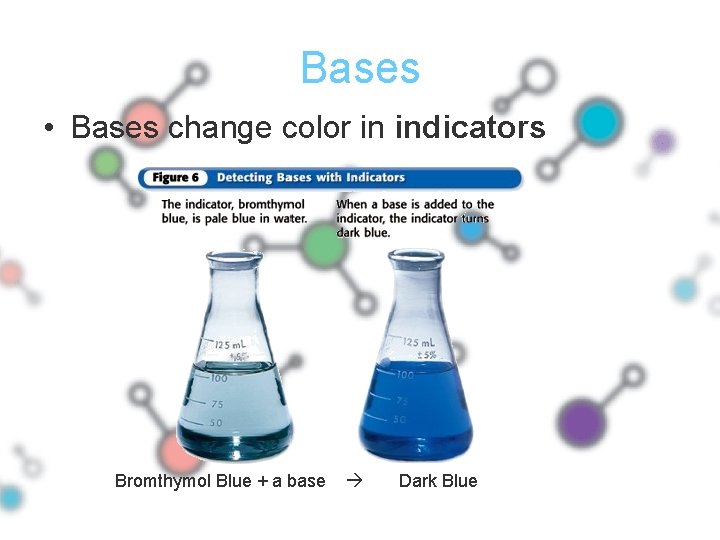
Bases • Bases change color in indicators Bromthymol Blue + a base Dark Blue
Bases • Because bases increase OH- ions, bases are conductors of electric currents.
Uses of bases • Na. OH – Make soap – Paper – Oven cleaner – Unclog sinks • Magnesium Hydroxide/Aluminum Hydroxide • antacids
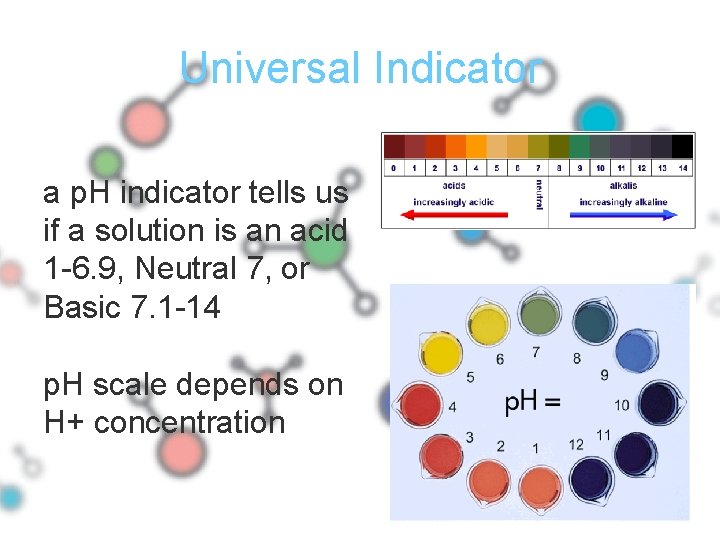
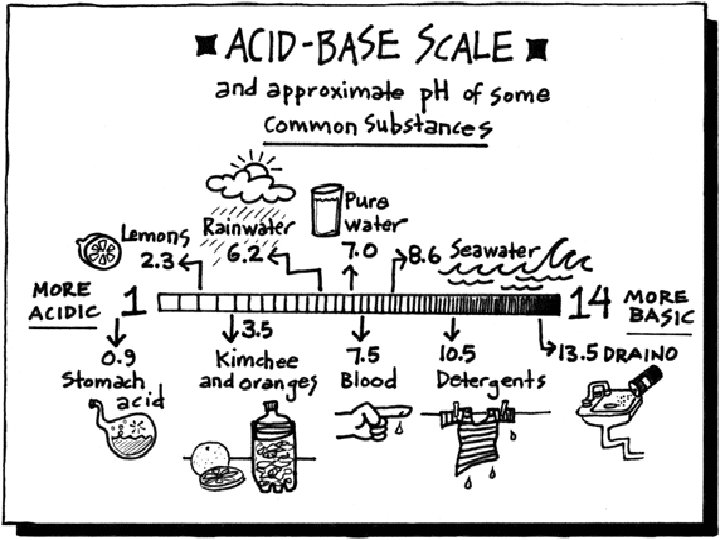
Universal Indicator a p. H indicator tells us if a solution is an acid 1 -6. 9, Neutral 7, or Basic 7. 1 -14 p. H scale depends on H+ concentration
- Slides: 16