3 1 Compounds A compound a pure substance
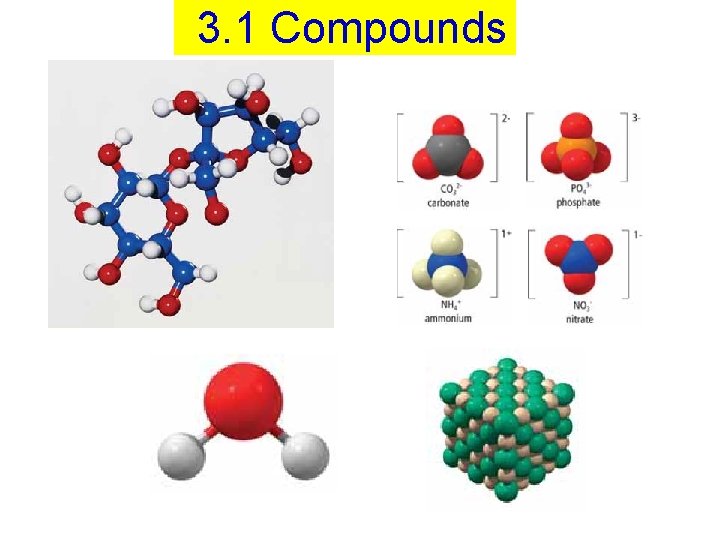
3. 1 Compounds
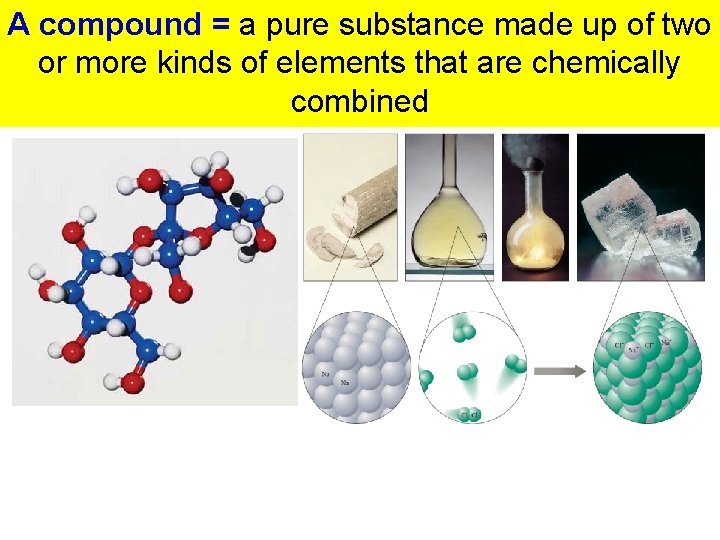
A compound = a pure substance made up of two or more kinds of elements that are chemically combined
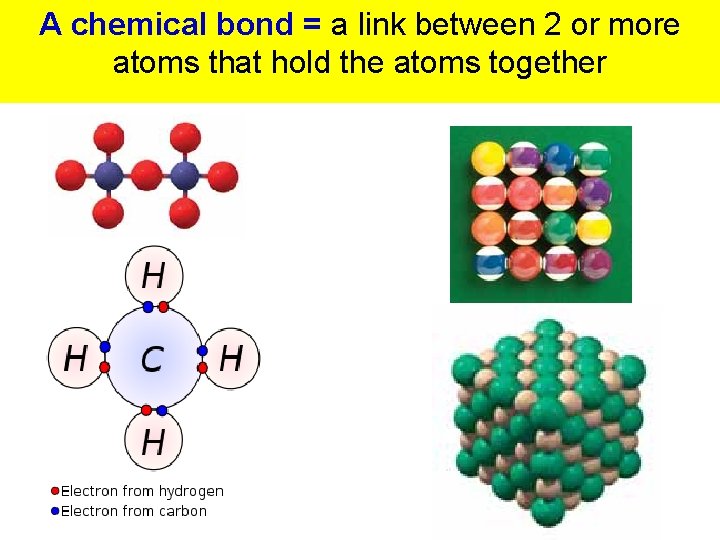
A chemical bond = a link between 2 or more atoms that hold the atoms together
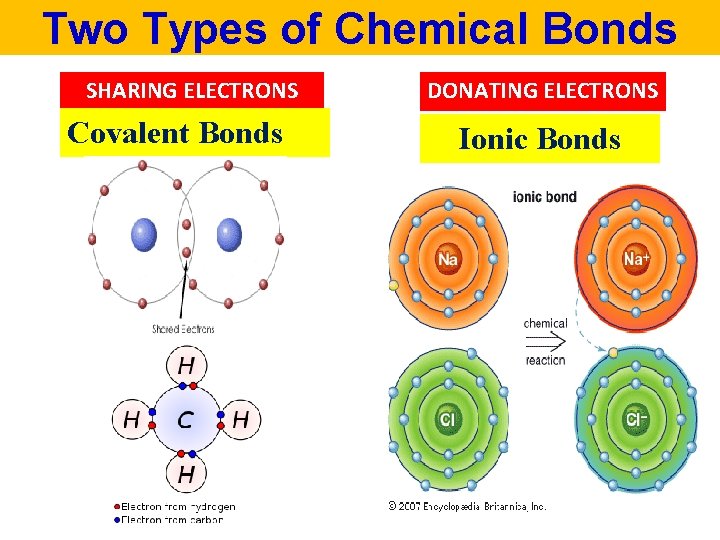
Two Types of Chemical Bonds SHARING ELECTRONS Covalent Bonds DONATING ELECTRONS Ionic Bonds
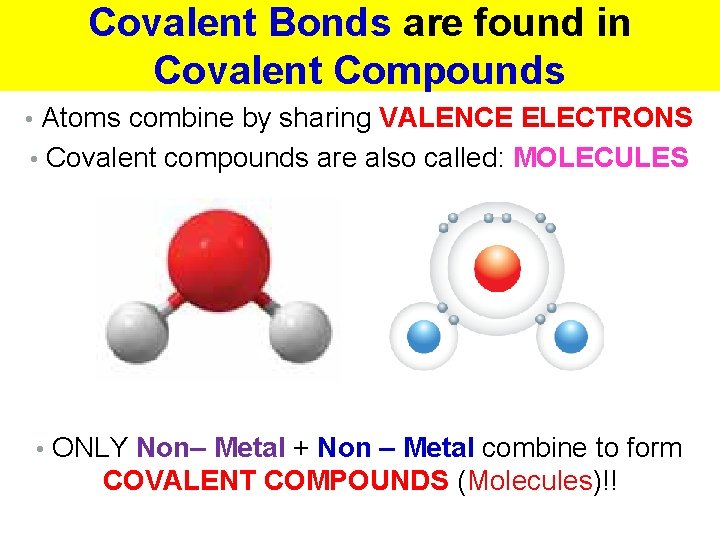
Covalent Bonds are found in Covalent Compounds • Atoms combine by sharing VALENCE ELECTRONS • Covalent compounds are also called: MOLECULES • ONLY Non– Metal + Non – Metal combine to form COVALENT COMPOUNDS (Molecules)!!
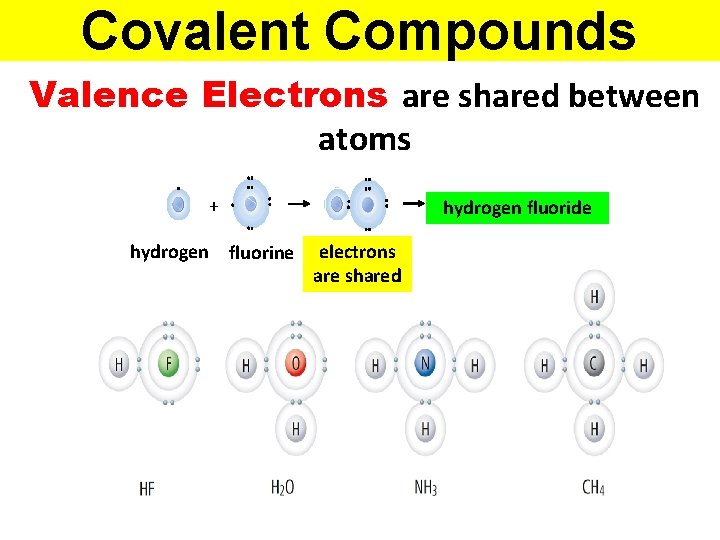
Covalent Compounds Valence Electrons are shared between atoms + hydrogen fluorine hydrogen fluoride electrons are shared
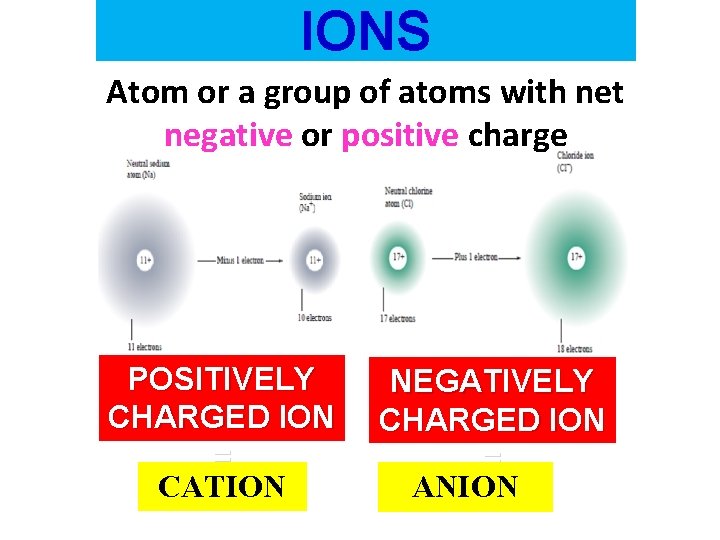
IONS Atom or a group of atoms with net negative or positive charge POSITIVELY CHARGED ION = CATION NEGATIVELY CHARGED ION = ANION
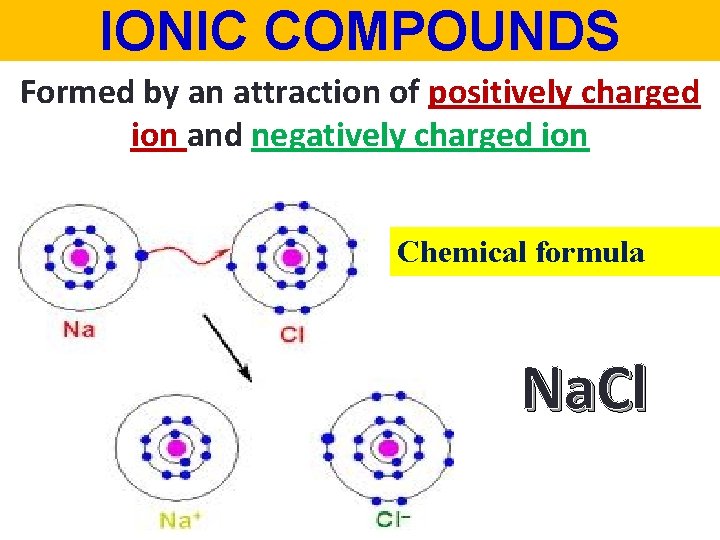
IONIC COMPOUNDS Formed by an attraction of positively charged ion and negatively charged ion Chemical formula Na. Cl
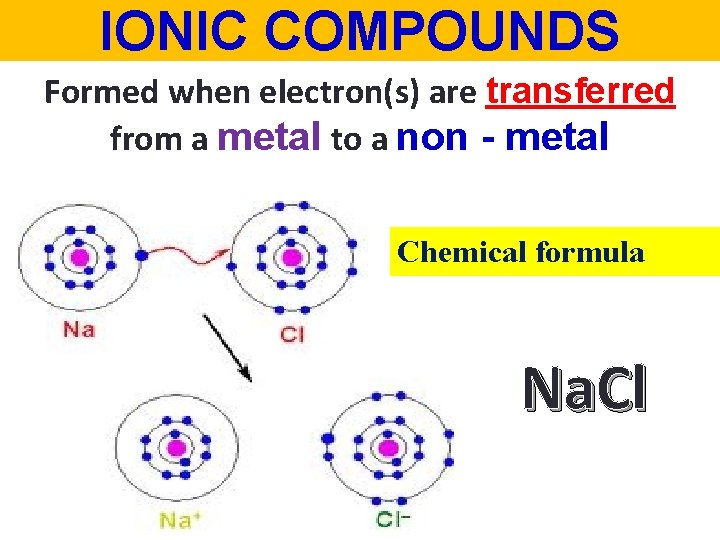
IONIC COMPOUNDS Formed when electron(s) are transferred from a metal to a non - metal Chemical formula Na. Cl

IONS Cations and Anions are attracted to each other IONIC BONDING Na. C l
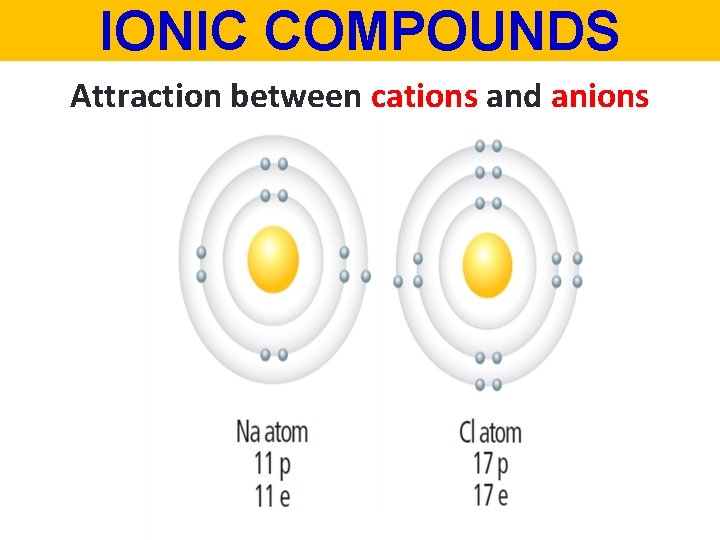
IONIC COMPOUNDS Attraction between cations and anions
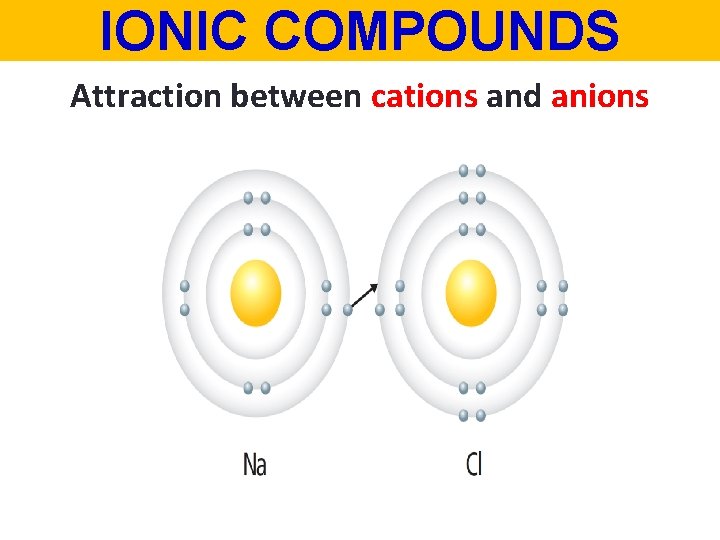
IONIC COMPOUNDS Attraction between cations and anions
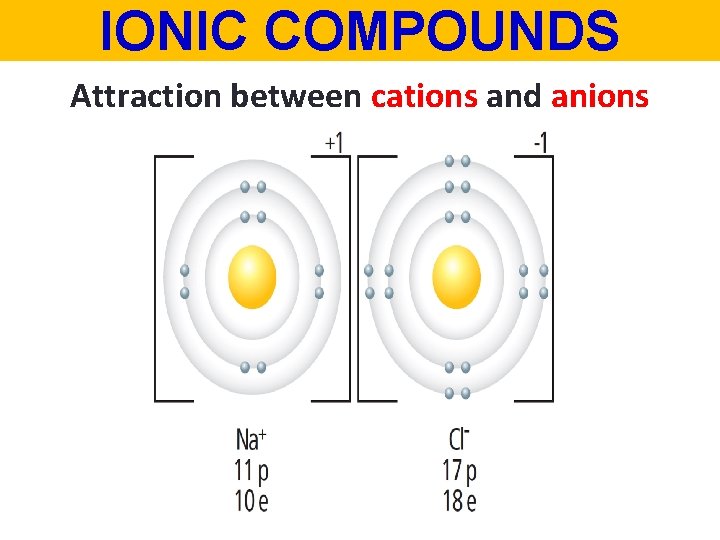
IONIC COMPOUNDS Attraction between cations and anions
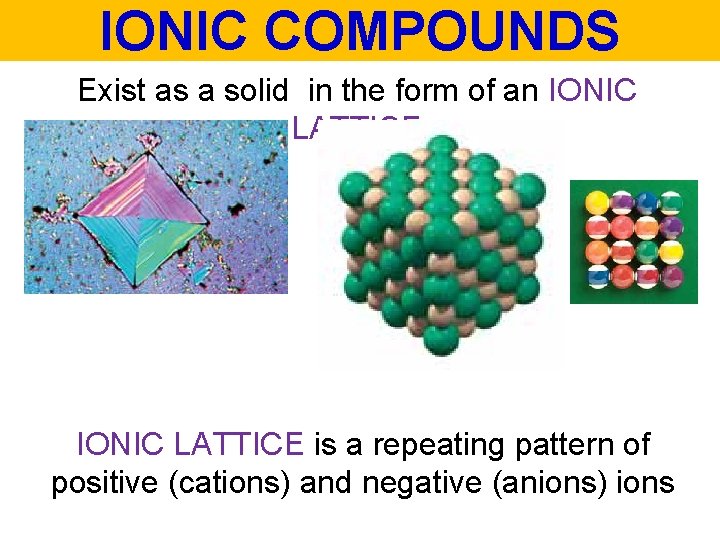
IONIC COMPOUNDS Exist as a solid in the form of an IONIC LATTICE is a repeating pattern of positive (cations) and negative (anions) ions

READING CHECK Page 79

BLM 1 – 30 Chemical Bonds Concept map
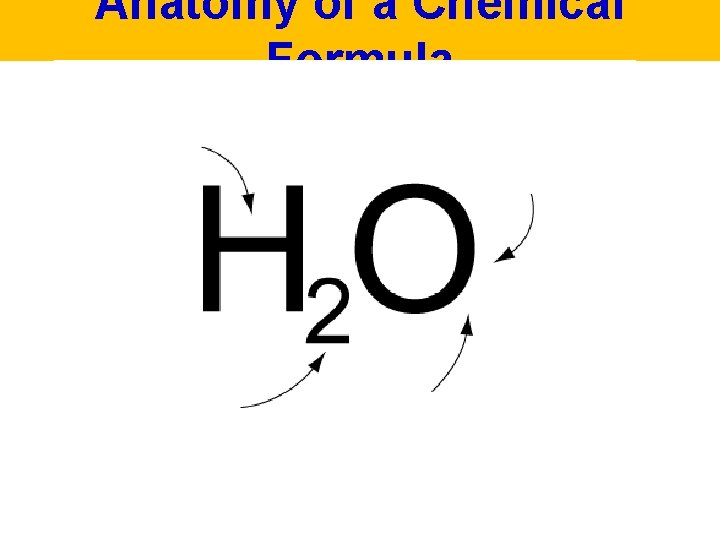
Anatomy of a Chemical Formula
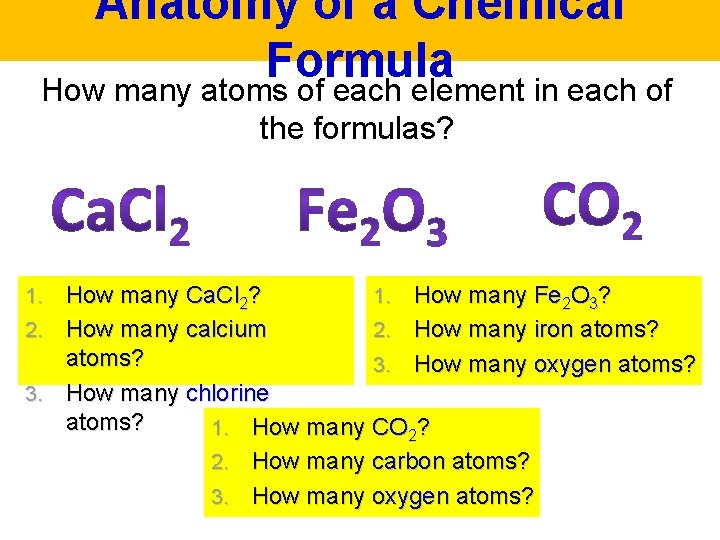
Anatomy of a Chemical Formula How many atoms of each element in each of the formulas? How many Ca. Cl 2? 1. How many Fe 2 O 3? 2. How many calcium 2. How many iron atoms? 3. How many oxygen atoms? 3. How many chlorine atoms? 1. How many CO 2? 2. How many carbon atoms? 3. How many oxygen atoms? 1.

BLM 1 - 30
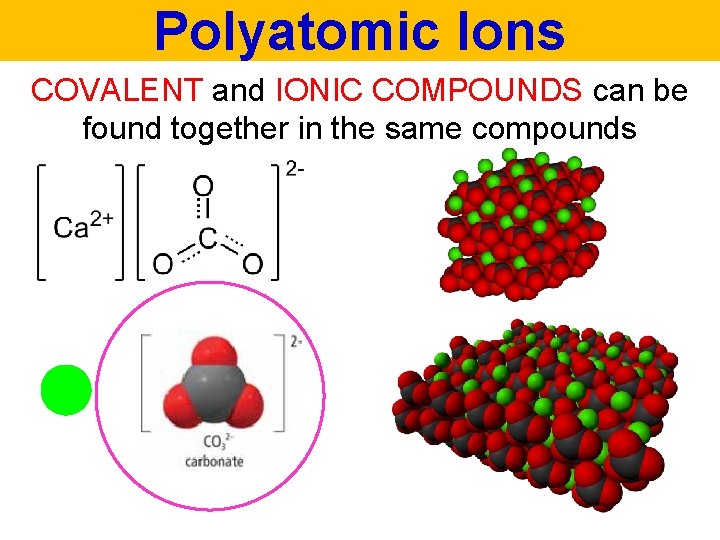
Polyatomic Ions COVALENT and IONIC COMPOUNDS can be found together in the same compounds
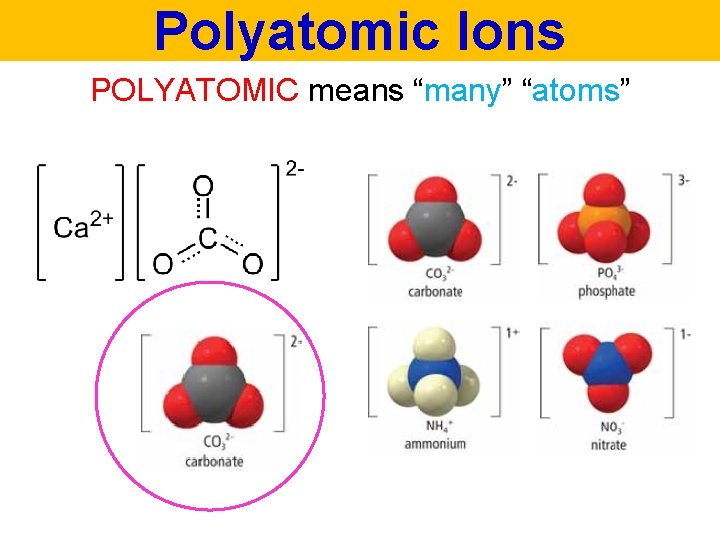
Polyatomic Ions POLYATOMIC means “many” “atoms”

READING CHECK Page 80

Making copper(II)chloride http: //www. paulslab. com/experiments/makingcopper-chloride. html
- Slides: 23