29 ACIDS BASES and p H Acids Bases

#29 ACIDS, BASES, and p. H
Acids, Bases, and p. H Vocabulary n Solution n Acid n Base n p. H scale n Ion- an atom or molecule with a positive (+) or negative (-) charge 1/3/2022 Template copyright 2005 www. brainybetty. com 2

Solutions n A solution is also called a homogenous mixture, which is a mixture of two or more substances that is identical throughout. n Many solutions have certain properties that make them an acid or a base 1/3/2022 Template copyright 2005 www. brainybetty. com 3

Acids are found in a your medicine cabinet, refrigerator, and on your kitchen shelves n Examples of acids include: aspirin, vitamin C, eyewash, oranges, grapes, lemons, grapefruit , apples, milk, tea, pickles, vinegar and carbonated drinks n

Why are acids important? Food digestion! You could not digest food if it were not for gastric acid in your stomach. n The manufacture of dyes, synthetic fibers, fertilizers, and explosives involves the use of acids. n

Acids n n n 1/3/2022 Acids taste sour when dissolved in water Produce a burning or prickly feeling on the skin Acids react with carbonates (like baking soda) and most metals Acids have a p. H of less than 7 Produce hydrogen ions (H+) when dissolved in water Examples of acids can be found in many foods such as; oranges, tomatoes, vinegar, and lemons. Template copyright 2005 www. brainybetty. com 6

Bases are also found in household products. n Examples of bases include: milk of magnesia, deodorants, ammonia and soap. n
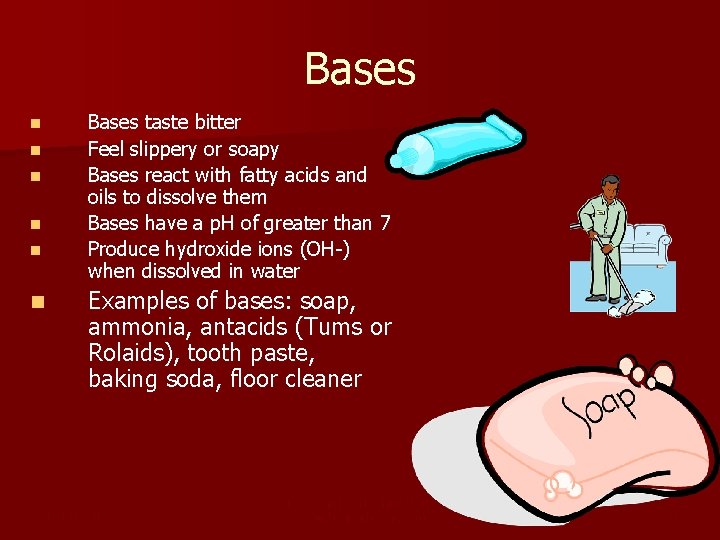
Bases taste bitter Feel slippery or soapy Bases react with fatty acids and oils to dissolve them Bases have a p. H of greater than 7 Produce hydroxide ions (OH-) when dissolved in water n n n Examples of bases: soap, ammonia, antacids (Tums or Rolaids), tooth paste, baking soda, floor cleaner 1/3/2022 Template copyright 2005 www. brainybetty. com 8

p. H scale § The p. H scale measures how acidic or basic a solution is, on a scale of 0 -14 and is a measurement of acidity. § p. H stands for “potential of hydrogen”, which refers to the ions acids and bases release when dissolved in water. The ions create a chemical reaction when in contact with a p. H indicator like red cabbage juice or p. H paper, which turns the indicator a different color. § Acids have a p. H below 7. The lower the p. H, the stronger the acid as that substance will release more hydrogen ions when dissolved. § Bases have a p. H above 7. The higher the p. H, the stronger the base, as that substance will also release more hydroxide ions when dissolved. 1/3/2022 Template copyright 2005 www. brainybetty. com 9

Neutrals n. A substance is considered neutral if it has a p. H of 7. n Neutrals are neither an acid or a base. n Example: Distilled (pure) water. n When acids and bases combine, they chemically react and destroy each other’s chemical properties. This is called neutralization.
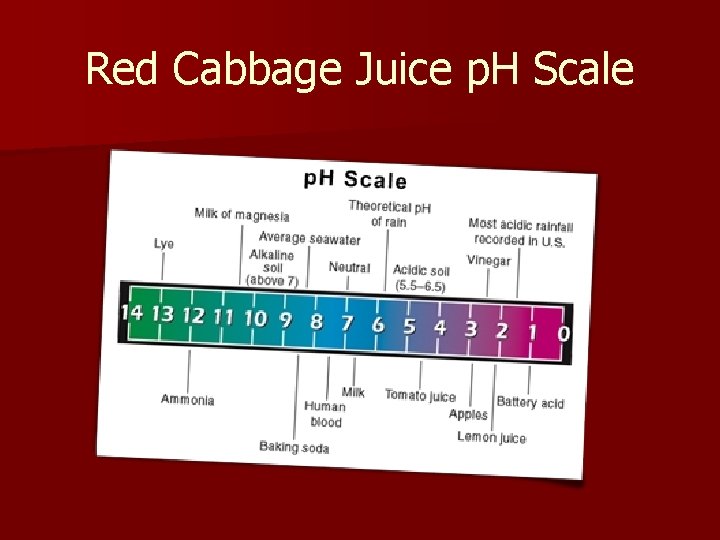
Red Cabbage Juice p. H Scale
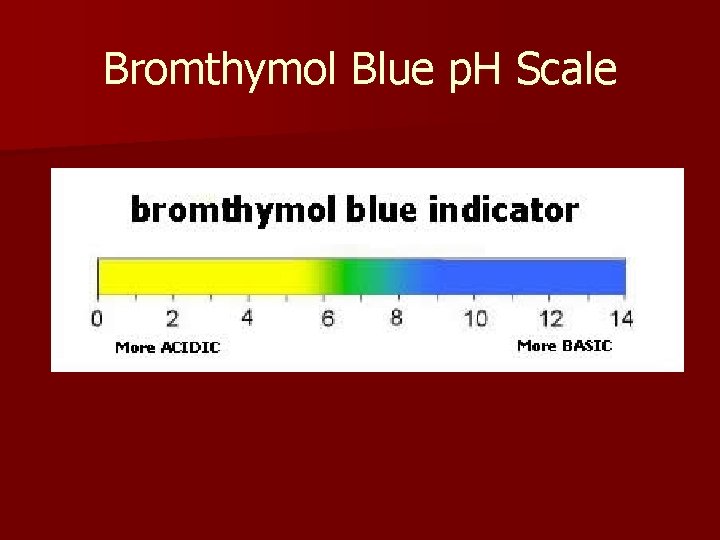
Bromthymol Blue p. H Scale
- Slides: 12