27 7 Potential energy surface Can be constructed
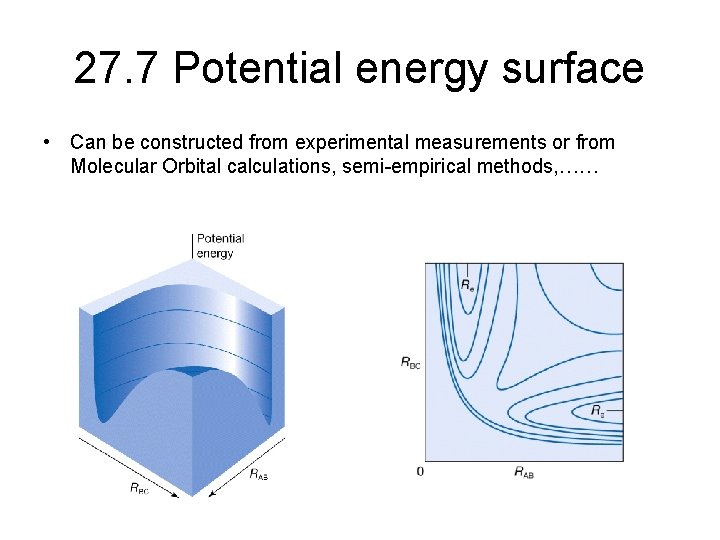
27. 7 Potential energy surface • Can be constructed from experimental measurements or from Molecular Orbital calculations, semi-empirical methods, ……
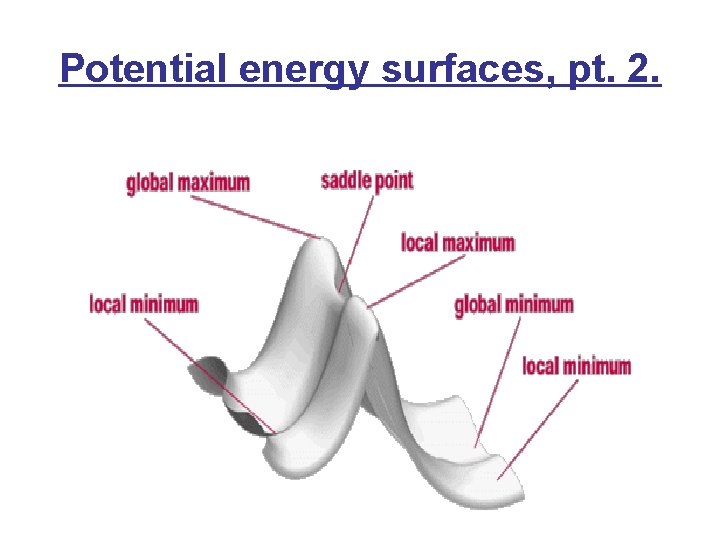
Potential energy surfaces, pt. 2.
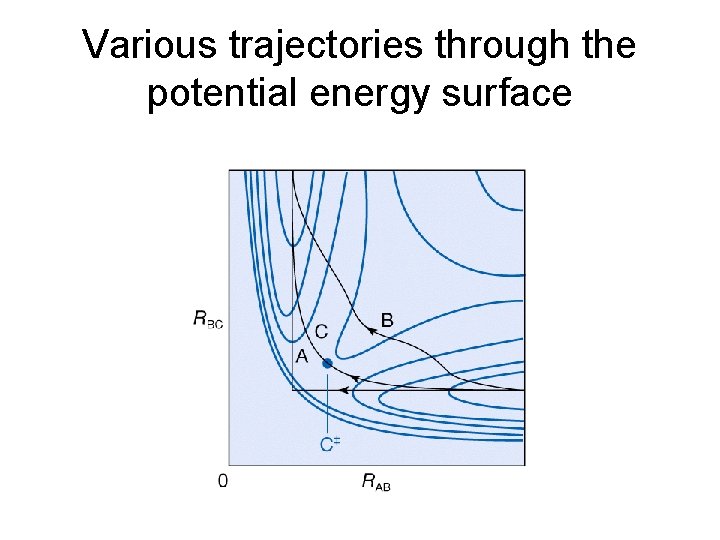
Various trajectories through the potential energy surface
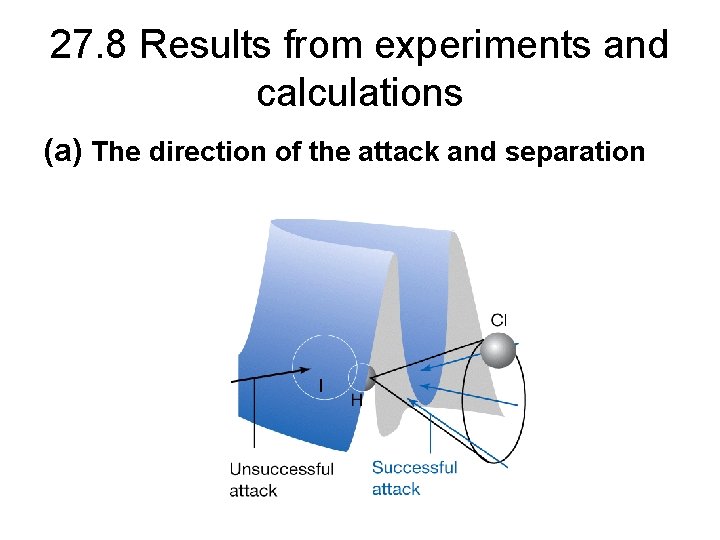
27. 8 Results from experiments and calculations (a) The direction of the attack and separation
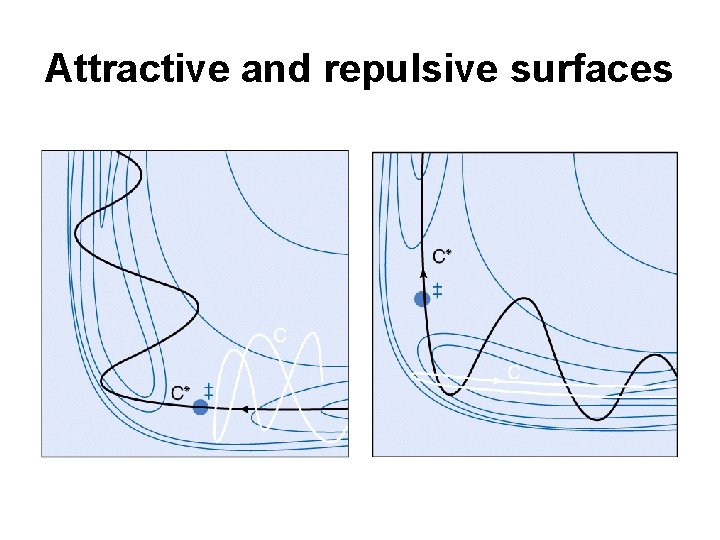
Attractive and repulsive surfaces
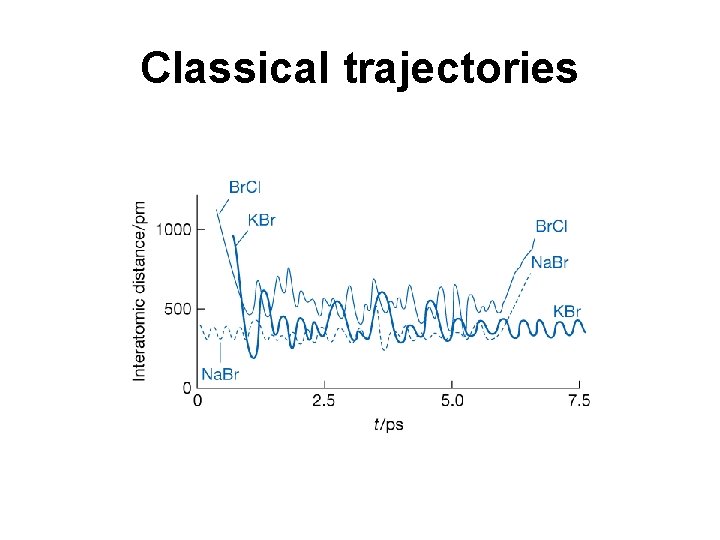
Classical trajectories
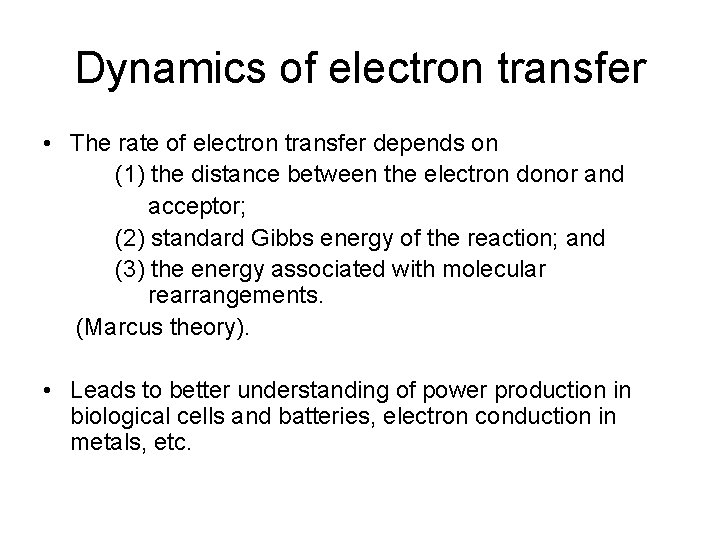
Dynamics of electron transfer • The rate of electron transfer depends on (1) the distance between the electron donor and acceptor; (2) standard Gibbs energy of the reaction; and (3) the energy associated with molecular rearrangements. (Marcus theory). • Leads to better understanding of power production in biological cells and batteries, electron conduction in metals, etc.
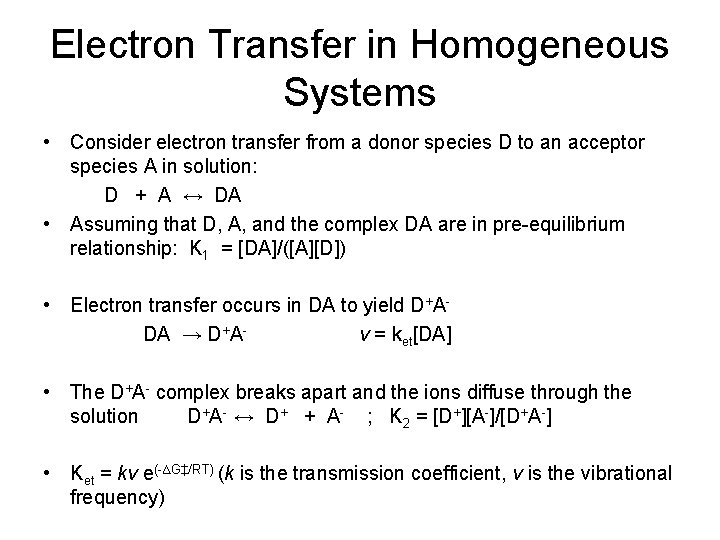
Electron Transfer in Homogeneous Systems • Consider electron transfer from a donor species D to an acceptor species A in solution: D + A ↔ DA • Assuming that D, A, and the complex DA are in pre-equilibrium relationship: K 1 = [DA]/([A][D]) • Electron transfer occurs in DA to yield D+ADA → D+Av = ket[DA] • The D+A- complex breaks apart and the ions diffuse through the solution D+A- ↔ D+ + A- ; K 2 = [D+][A-]/[D+A-] • Ket = kv e(-∆G‡/RT) (k is the transmission coefficient, v is the vibrational frequency)

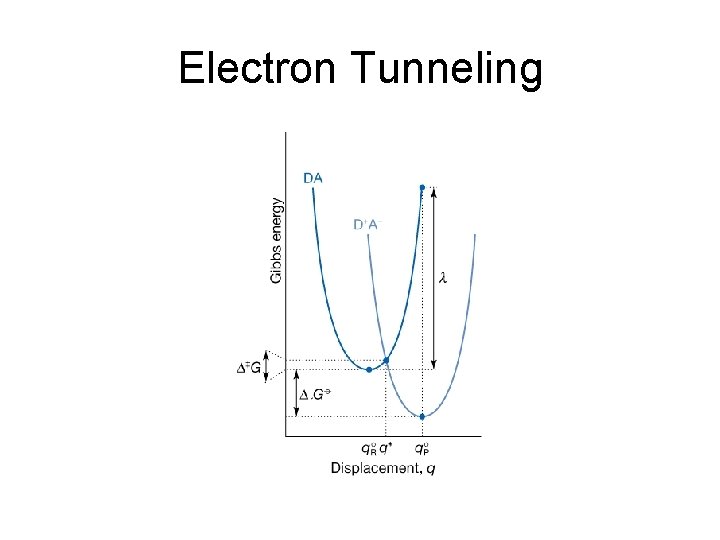
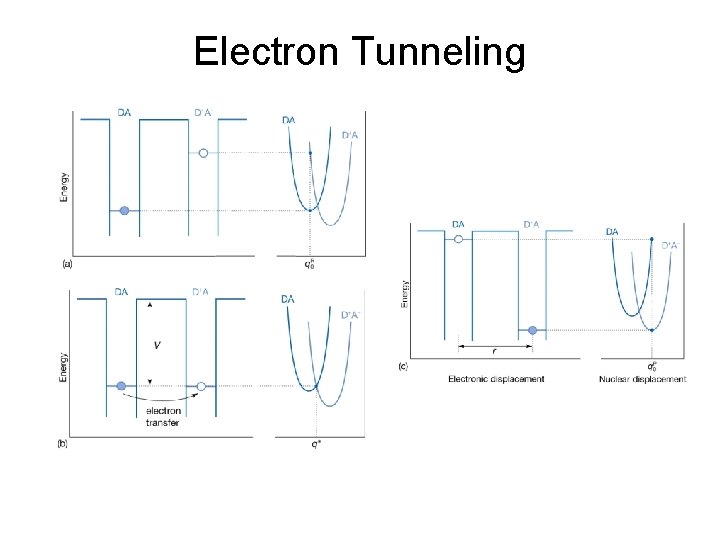
29. 1 Electron transfer theory Two key aspects: • Electrons are transferred by tunneling through a potential energy barrier, the height of which is partly determined by the ioinzation energies of the DA and D+A- complexes. • The complex DA and the solvent molecules surrounding it undergo structural rearrangements prior to electron transfer. The energy associated with these rearrangements and the standard reaction Gibbs energy determine ∆G‡
Electron Tunneling
Electron Tunneling

The Gibbs energy of activation • Λ is the re-organization energy
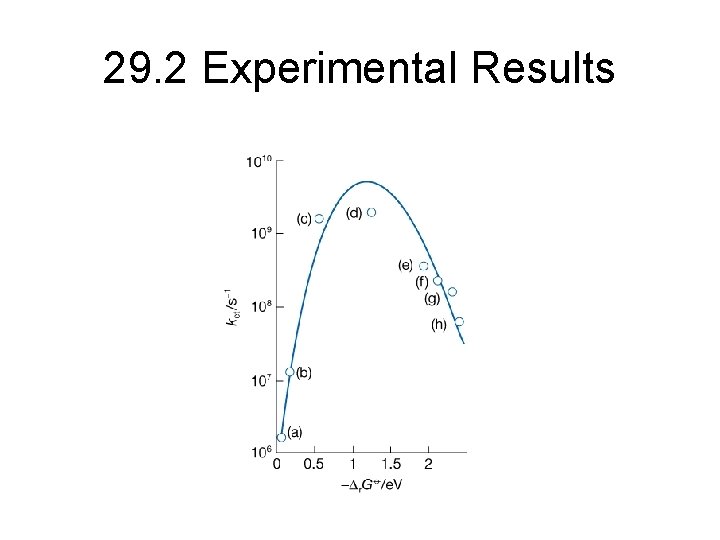
29. 2 Experimental Results
- Slides: 13