27 301 MicrostructureProperties Lecture 2 Recrystallization Theoretical Practical






- Slides: 25

27 -301 Microstructure-Properties Lecture 2: Recrystallization Theoretical & Practical Aspects Profs. A. D. Rollett, M. De Graef Updated 27 th September, 2015

2 Objectives • The main objective of this lecture is to introduce you to the process of recrystallization and to prepare you for a laboratory exercise on this topic. • You will have mastered the material in this lecture if you can describe the process in qualitative terms, can relate it to thermomechanical processing in general and know how to apply Johnson-Mehl-Avrami-Kolmogorov analysis to the kinetics. • The use of the JMAK equation for analysis of phase transformations was covered in 27 -217. Please acknowledge Carnegie Mellon if you make public use of these slides

3 Q&A • • What microstructural evolution (change) occurs during recrystallization? Deformed (dislocated) material is replaced by dislocation-free material by the long-range motion of grain boundaries. Is recrystallization thermally activated? Yes, the long-range motion of grain boundaries is thermally activated, which means that recrystallization is also. What do we measure in order to apply the JMAK Eq to recrystallization? We measure the fraction recrystallized, either by area in micrographs or by fractional change in hardness. Why is the nucleation of recrystallization necessarily heterogeneous? If we apply classical nucleation theory we discover that the available driving force is too small and the interfacial energy is too high, such that the critical radius of a nucleus would be or order 1 micron. • • How can we estimate the driving force for recrystallization? We can estimate the dislocation density from the flow stress (during the prior deformation) and convert that to a stored energy. How does the recrystallized grain size vary with prior strain? Grain size decreases with increasing strain, although the largest change occurs at small strains. What are the requirements for a new grain to grow in recrystallization? The new grain must be dislocation free and it must have a mobile perimeter, which means it must be bordered by a high angle boundary. What effect does solute have on recrystallization? Solutes exert a drag on moving grain boundaries therefore recrystallization is slower (or requires high temperatures) for higher solute levels. Please acknowledge Carnegie Mellon if you make public use of these slides

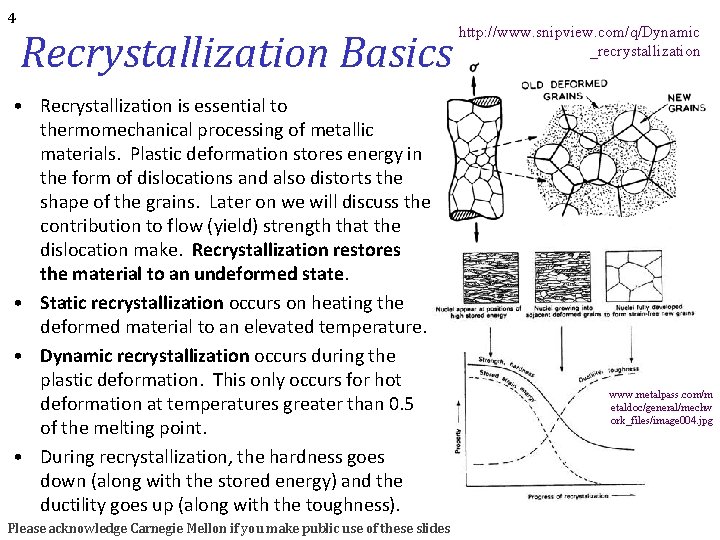
4 Recrystallization Basics • Recrystallization is essential to thermomechanical processing of metallic materials. Plastic deformation stores energy in the form of dislocations and also distorts the shape of the grains. Later on we will discuss the contribution to flow (yield) strength that the dislocation make. Recrystallization restores the material to an undeformed state. • Static recrystallization occurs on heating the deformed material to an elevated temperature. • Dynamic recrystallization occurs during the plastic deformation. This only occurs for hot deformation at temperatures greater than 0. 5 of the melting point. • During recrystallization, the hardness goes down (along with the stored energy) and the ductility goes up (along with the toughness). Please acknowledge Carnegie Mellon if you make public use of these slides http: //www. snipview. com/q/Dynamic _recrystallization www. metalpass. com/m etaldoc/general/mechw ork_files/image 004. jpg

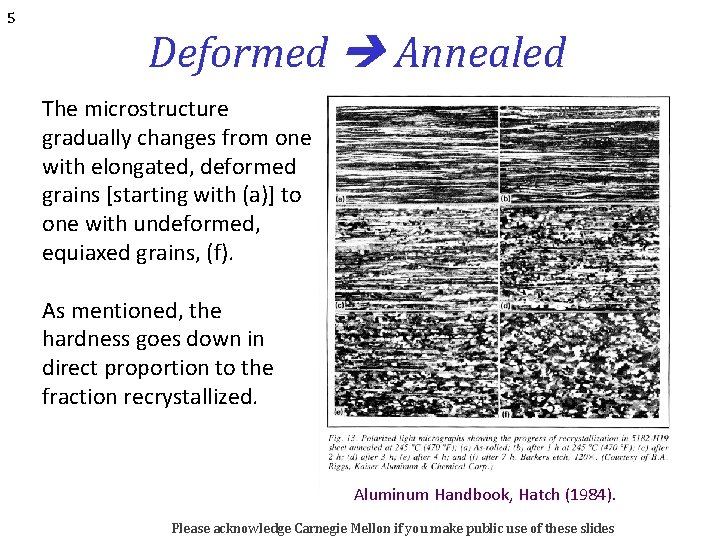
5 Deformed Annealed The microstructure gradually changes from one with elongated, deformed grains [starting with (a)] to one with undeformed, equiaxed grains, (f). As mentioned, the hardness goes down in direct proportion to the fraction recrystallized. Aluminum Handbook, Hatch (1984). Please acknowledge Carnegie Mellon if you make public use of these slides

6 Annealing Processes • • • Recrystallization is one example of a process that occurs during annealing of materials. Annealing is simply the exposure of a material to elevated temperature for a specified period of time. Various thermally activated processes occur during annealing that the materials engineer seeks to control in order to optimize properties. Other processes include precipitation, phase transformation, recovery, grain growth, carburization, and sintering. Primary recrystallization means that the process is driven by stored energy: if you see recrystallization without qualification, it is primary recrystallization. Secondary recrystallization is sometimes used to signify curvature-driven grain growth (as the process that follows primary recrystallization); in industrial processing, it often implies abnormal grain growth, during which only a minority of grains grow to sizes much larger than the initial. Occasionally you will see reference to Tertiary recrystallization, which is a further stage of grain growth. Both secondary and tertiary grain growth can be driven by surface energy, or magnetic energy, in addition to the excess free energy of grain boundaries. Recovery is the decrease of dislocation density that occurs by motion and annihilation of individual dislocations. Grain growth is the coarsening of the grain structure by motion of grain boundaries. The driving force is the motion of boundaries due to their curvature, so overall, the driving force is the reduction in total area of boundary. Carburization is an example of a change of chemical composition near the surface brought about by the presence of a high chemical potential for carbon (e. g. by having CO in the furnace atmosphere) during annealing. Nitriding is an equivalent process using nitrogen. This is important in steels for producing high hardness at the surface of a material. See DOI: 10. 4236/jmmce. 2012. 111002 for an environmentally friendly approach. Please acknowledge Carnegie Mellon if you make public use of these slides

7 Recrystallization: mechanisms • The basic mechanism of (primary) recrystallization is the (long-range) motion of grain boundaries that removes dislocation density from the material. • A consequence of the requirement for long-range boundary migration is that recrystallization is a thermally activated process. • In most materials, temperatures > Tm/3 are required for recrystallization to proceed at a measurable rate. • Why? Grain boundaries are slowed down by the presence of solute and most practical materials have significant amounts of solute. • Solute refers to any dissolved element and can be either a deliberate alloying addition, or an impurity. Please acknowledge Carnegie Mellon if you make public use of these slides

8 Recrystallization: measurement • How can we measure recrystallization? • The traditional method is to perform optical metallography on sectioned samples. Recrystallized grains appear as approximately equiaxed grains with uniform color (see figures on previous slide). Unrecrystallized grains appear as deformed grains with irregular contrast, generally nonequiaxed (reflecting the overall change in shape). Examples were shown in previous slides. • We measure the area fraction (AA) of recrystallized versus unrecrystallized material. Stereology tells us that this area fraction is equivalent to the volume fraction (VV) of recrystallized material. • The most efficient way to quantify the progress of recrystallization is to measure hardness which decreases with time. The assumption is that the fractional change in hardness is equal to the fraction recrystallized. Please acknowledge Carnegie Mellon if you make public use of these slides

9 Recrystallization Characteristics • In order for the boundary between a new grain (nucleus) and the deformed material to be able to move, it must be a high angle boundary. This is a consequence of the properties of boundaries, see L 1. • The requirement that new grains have high angle boundaries means that the final grain size and the rate at which recrystallization takes place is highly dependent on the strain level. • Higher strains mean greater lattice rotations (from dislocation slip) inside grains and higher stored energies. Therefore the probability of generating new grains increases with strain and the driving force increases. • Increasing probability for nucleation translates directly into increased density of nuclei and therefore smaller recrystallized grain size. Please acknowledge Carnegie Mellon if you make public use of these slides

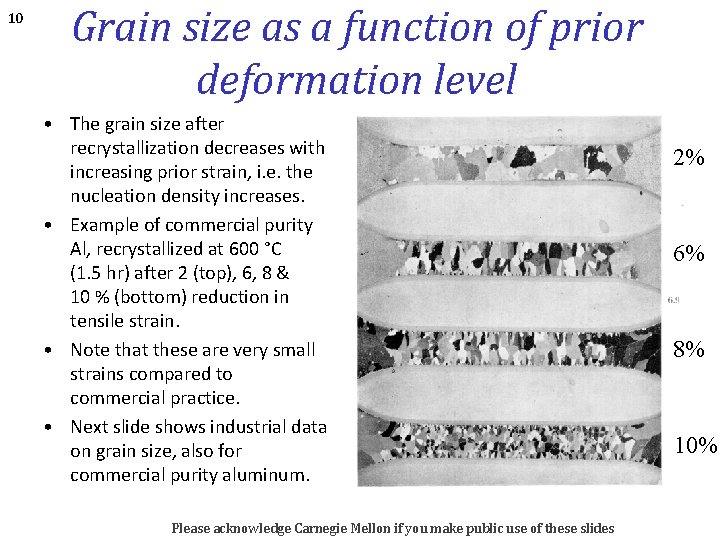
10 Grain size as a function of prior deformation level • The grain size after recrystallization decreases with increasing prior strain, i. e. the nucleation density increases. • Example of commercial purity Al, recrystallized at 600 °C (1. 5 hr) after 2 (top), 6, 8 & 10 % (bottom) reduction in tensile strain. • Note that these are very small strains compared to commercial practice. • Next slide shows industrial data on grain size, also for commercial purity aluminum. Please acknowledge Carnegie Mellon if you make public use of these slides 2% 6% 8% 10%

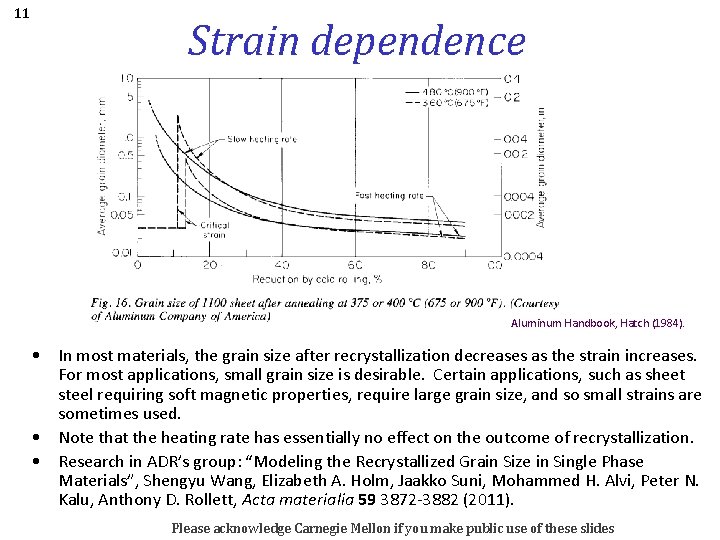
11 Strain dependence Aluminum Handbook, Hatch (1984). • In most materials, the grain size after recrystallization decreases as the strain increases. For most applications, small grain size is desirable. Certain applications, such as sheet steel requiring soft magnetic properties, require large grain size, and so small strains are sometimes used. • Note that the heating rate has essentially no effect on the outcome of recrystallization. • Research in ADR’s group: “Modeling the Recrystallized Grain Size in Single Phase Materials”, Shengyu Wang, Elizabeth A. Holm, Jaakko Suni, Mohammed H. Alvi, Peter N. Kalu, Anthony D. Rollett, Acta materialia 59 3872 -3882 (2011). Please acknowledge Carnegie Mellon if you make public use of these slides

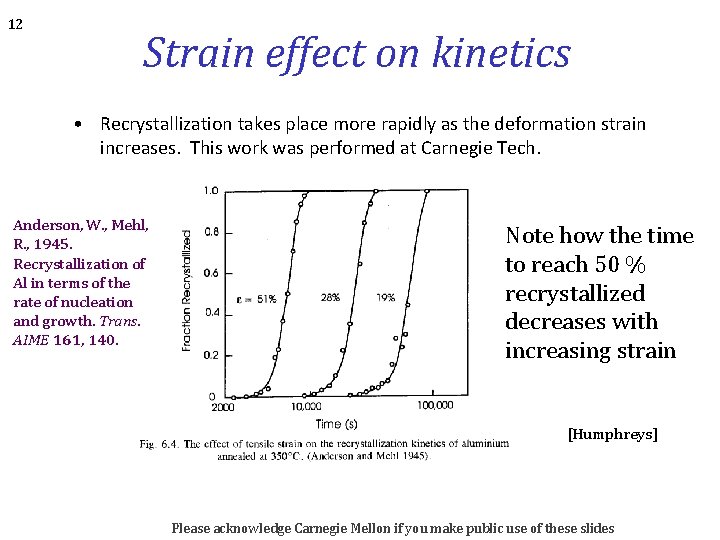
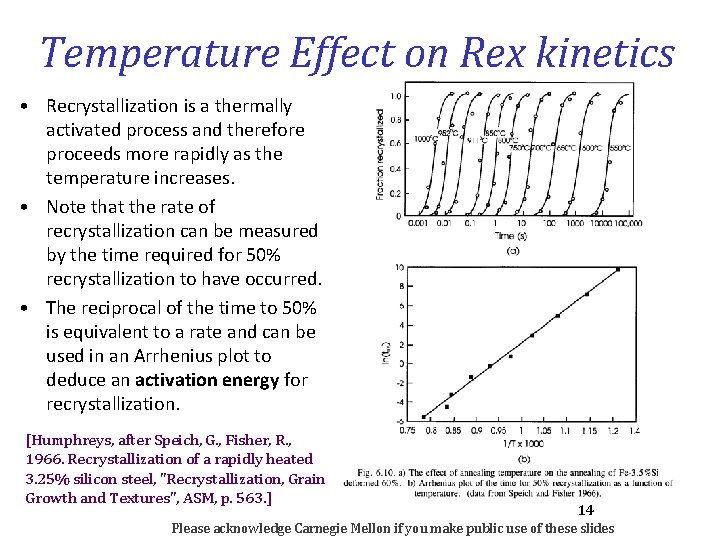
12 Strain effect on kinetics • Recrystallization takes place more rapidly as the deformation strain increases. This work was performed at Carnegie Tech. Anderson, W. , Mehl, R. , 1945. Recrystallization of Al in terms of the rate of nucleation and growth. Trans. AIME 161, 140. Note how the time to reach 50 % recrystallized decreases with increasing strain [Humphreys] Please acknowledge Carnegie Mellon if you make public use of these slides

13 Temperature dependence • The growth of new grains requires motion of grain boundaries. Boundary migration occurs by the transfer of atoms across the boundary which is a diffusion-like process, i. e. thermally activated. • Solutes have a strong effect on boundaries because the interaction leads to segregation (generally an excess of solute on the boundary). In effect, moving the boundary forces the solute to move with it. • A suitable measure of the “reaction rate” is the time for 50% recrystallization. Please acknowledge Carnegie Mellon if you make public use of these slides

Temperature Effect on Rex kinetics • Recrystallization is a thermally activated process and therefore proceeds more rapidly as the temperature increases. • Note that the rate of recrystallization can be measured by the time required for 50% recrystallization to have occurred. • The reciprocal of the time to 50% is equivalent to a rate and can be used in an Arrhenius plot to deduce an activation energy for recrystallization. [Humphreys, after Speich, G. , Fisher, R. , 1966. Recrystallization of a rapidly heated 3. 25% silicon steel, "Recrystallization, Grain Growth and Textures", ASM, p. 563. ] 14 Please acknowledge Carnegie Mellon if you make public use of these slides

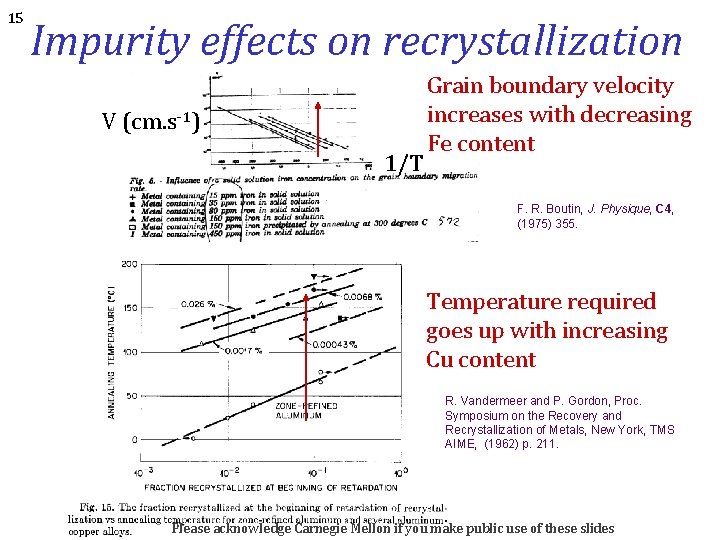
15 Impurity effects on recrystallization V (cm. s-1) 1/T Grain boundary velocity increases with decreasing Fe content F. R. Boutin, J. Physique, C 4, (1975) 355. Temperature required goes up with increasing Cu content R. Vandermeer and P. Gordon, Proc. Symposium on the Recovery and Recrystallization of Metals, New York, TMS AIME, (1962) p. 211. Please acknowledge Carnegie Mellon if you make public use of these slides

16 Nucleation & Growth • Based on the microstructural characteristics (a different type of material appears as dispersed ‘particles’ and grows to the point of replacing the deformed material), recrystallization is classified as a ‘nucleation & growth’ phenomenon. • Although treating recrystallization as a nucleation & growth process is perfectly adequate, more detailed examination shows that it is actually a continuous coarsening process. The coarsening is, however, so highly heterogeneous that classification depends on the length scale at which it is characterized. • The clearest explanation of why recrystallization must be heterogeneous, and not homogeneous (like a precipitation reaction), requires an analysis of the driving force available and what would be the size of the critical nucleus (see the discussion in the book by Martin, Doherty & Cantor). The latter value turns out to be far too large for homogeneous nucleation to be viable. See following slides for an explanation. Please acknowledge Carnegie Mellon if you make public use of these slides

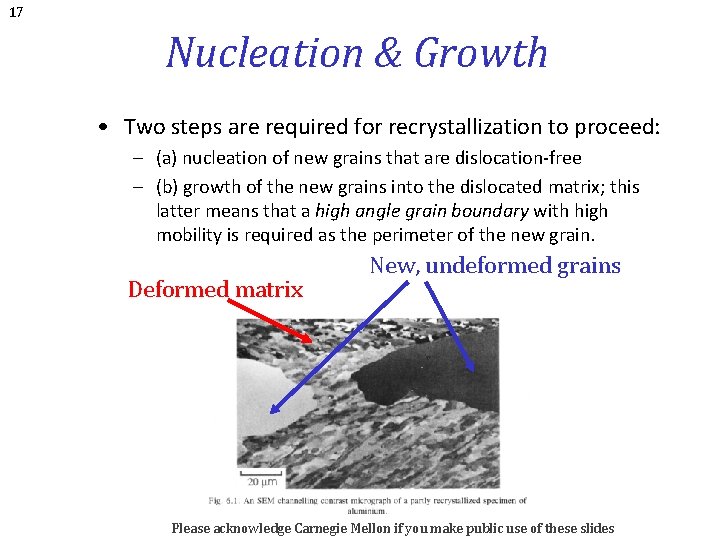
17 Nucleation & Growth • Two steps are required for recrystallization to proceed: – (a) nucleation of new grains that are dislocation-free – (b) growth of the new grains into the dislocated matrix; this latter means that a high angle grain boundary with high mobility is required as the perimeter of the new grain. Deformed matrix New, undeformed grains Please acknowledge Carnegie Mellon if you make public use of these slides

18 Source of stored energy • Martin, Doherty & Cantor distinguish between microstructural changes driven by chemical energy and change driven by strain energy. • Recrystallization is a process of microstructural change driven by strain energy i. e. energy stored in the accumulated dislocation density. • We will examine the details of plastic deformation later in the course. For now, it is sufficient to know that plastic deformation requires a high level of dislocation activity on at least 5 slip systems in each grain. Dislocations intersect and multiply leaving behind a highly irregular structure. This storage of dislocation line length is the direct cause of work hardening and is the strain energy that drives recrystallization. When we refer to work hardening, this is equivalent to the increase in flow stress during, e. g. , a tensile test. Please acknowledge Carnegie Mellon if you make public use of these slides

19 Nucleation Issues • Nucleation in recrystallization must be a heterogeneous nucleation process because the driving force is too small to sustain homogeneous nucleation. • Estimate of driving force, E: – – – Energy per unit length of dislocation Gb 2 Thus energy/volume, E Gb 2 r Typical cold worked dislocation density, r = 1015. m-2 For Al, G = 27 GPa, b =0. 28 nm, E 2 J. m-3 ( = 2 MPa) For a HAGB energy (in Al) s = 0. 5 J. m-2, the critical nucleus size, rcrit = 2 s/E 0. 25µm, which is a very large critical radius. In fact this is too large for homogeneous nucleation to occur. • Therefore nucleation in recrystallization must be heterogeneous. • Note that the units of stress are the same as pressure, which are also equivalent to energy per unit volume Please acknowledge Carnegie Mellon if you make public use of these slides

20 Heterogeneous Nucleation • Nucleation of recrystallization therefore occurs on defects in the material. • Sites for nucleation: – Prior grain boundaries (strain induced boundary migration, SIBM) – Deformation bands or shear bands, i. e. regions of non-uniform rotation of the lattice – Coarsening (recovery) of a subgrain structure – Coarsening (recovery) of dislocation structure near to coarse particles; this is called Particle Stimulated Nucleation (PSN). Please acknowledge Carnegie Mellon if you make public use of these slides

21 Growth of New Grains in Recrystallization • The interface between a new grain and the deformed (unrecrystallized) material (“matrix”) is a grain boundary. • Grain boundaries vary in their mobility, M, i. e. the constant of proportionality between migration rate, v, and driving force, E. • Assume a linear relationship: v = ME. • The driving force (for recrystallization) is exactly the stored energy estimated on a previous slide. • High angle grain boundaries (HAGB), with a misorientation angle ∆q >15°, are typically far more mobile than low angle boundaries (LAGB), often by orders of magnitude. Please acknowledge Carnegie Mellon if you make public use of these slides

22 Laboratory 1 - Introduction • The objectives of the first Lab are as follows: – – – – – Demonstrate recrystallization Develop metallography skills Ability to measure grain size Ability to measure hardness Demonstrate effect of strain on recrystallized grain size Demonstrate effect of temperature on recrystallized grain size Demonstrate effect of strain on hardness Demonstrate effect of temperature on hardness Demonstrate the Hall-Petch effect Promote critical thinking about the reasons for the variations in grain size and hardness observed Please acknowledge Carnegie Mellon if you make public use of these slides

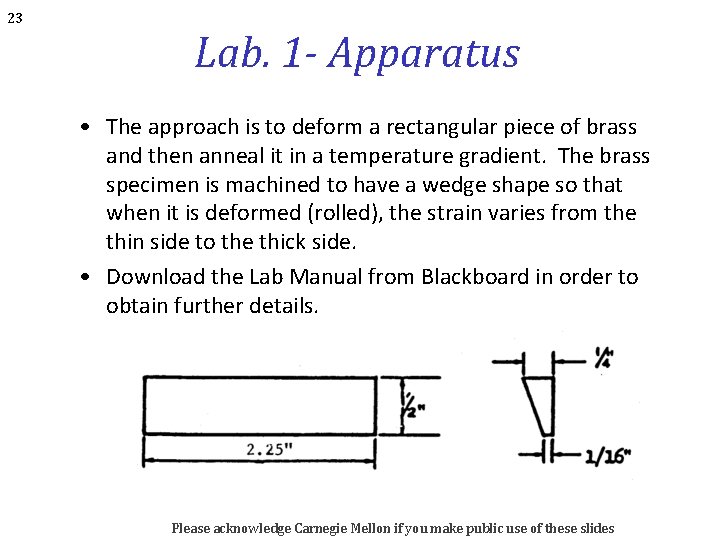
23 Lab. 1 - Apparatus • The approach is to deform a rectangular piece of brass and then anneal it in a temperature gradient. The brass specimen is machined to have a wedge shape so that when it is deformed (rolled), the strain varies from the thin side to the thick side. • Download the Lab Manual from Blackboard in order to obtain further details. Please acknowledge Carnegie Mellon if you make public use of these slides

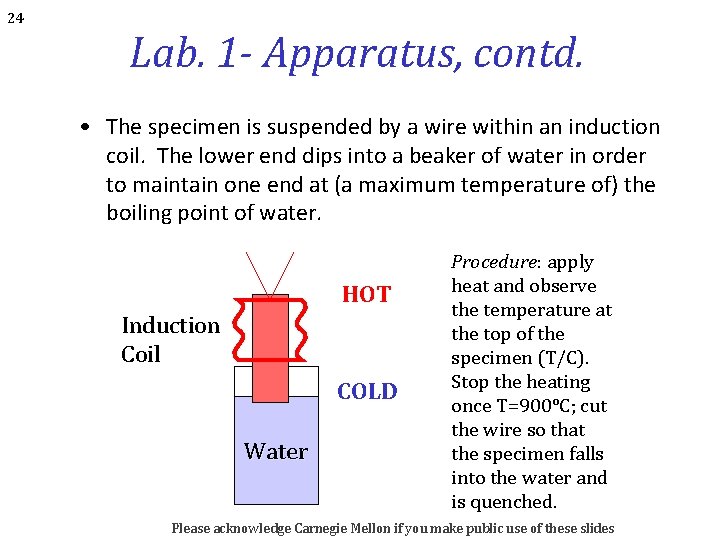
24 Lab. 1 - Apparatus, contd. • The specimen is suspended by a wire within an induction coil. The lower end dips into a beaker of water in order to maintain one end at (a maximum temperature of) the boiling point of water. HOT Induction Coil COLD Water Procedure: apply heat and observe the temperature at the top of the specimen (T/C). Stop the heating once T=900°C; cut the wire so that the specimen falls into the water and is quenched. Please acknowledge Carnegie Mellon if you make public use of these slides

25 Lab 1 - Expected Results • Hardness: before recrystallization, the hardness should increase with increasing strain (hint - relate this to what you know about stress-strain behavior in metals). • After recrystallization, the grain size will increase with increasing temperature, but decrease with increasing prior strain. • Also after recrystallization, the hardness will increase with decreasing grain size (Hall-Petch effect) in the recrystallized areas. • Note: a critical part of the Lab is to obtain high quality images of the grain structure so that you can measure grain size. The metallography involved requires skill and effort. Also, computer-based submissions are required (Word, or La. Te. X). Please acknowledge Carnegie Mellon if you make public use of these slides