26122021 Nuclear Reactions Radioactive Decay Summary Notes pages















- Slides: 18

26/12/2021 Nuclear Reactions Radioactive Decay (Summary Notes pages 18 -20) Particles and Waves M Ashton 2013

What are we learning today? �Revision of the structure of the atom. �Nuclear Symbols �What is meant by alpha, beta and gamma decay of radionuclides. �The processes occurring in nuclear reactions written in symbolic form.

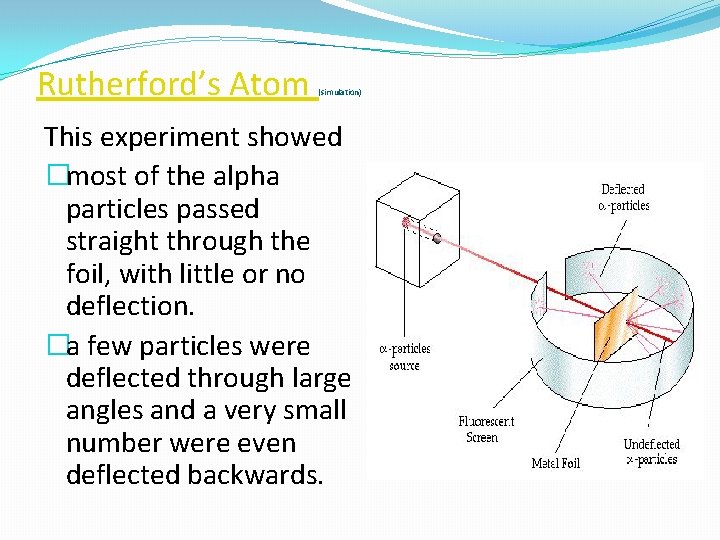
Rutherford’s Atom (simulation) This experiment showed �most of the alpha particles passed straight through the foil, with little or no deflection. �a few particles were deflected through large angles and a very small number were even deflected backwards.

Tap for simulation �The fact that most of the particles passed straight through the foil, which was at least 100 atoms thick, suggested that the atom must be mostly empty space. �To produce the large deflections the positively charged alpha particles must be encountering something very small, of very large mass and a positive charge.

Bohr’s Atom Bohr proposed that �the nucleus contains protons and neutrons and is positively charged. �electrons orbit the nucleus in distinct energy levels. electron proton neutron

Nuclear Symbols �We use shorthand to represent each element: Mass number Atomic number A Z X Element symbol �The mass number, A, tells us the total number of protons and neutrons in the nucleus. �The atomic number, Z, tells us how many protons.

e. g. This tells us there are 6 protons and 6 neutrons. 12 6 C This tells us there are 6 protons. This tells us the element is carbon.

What is an isotope? An isotope is an atom that has the same number of protons and electrons but a different number of neutrons. i. e. 12 13 14 6 6 6 C 6 electrons 6 protons 6 neutrons C 6 electrons 6 protons 7 neutrons C 6 electrons 6 protons 8 neutrons

Q 42 page 19

Radioactive Decay �This is the breakdown of a nucleus to release energy and matter. �This allows unstable nuclei to become stable. �There are three types of radiation that can be emitted: �alpha �beta �gamma


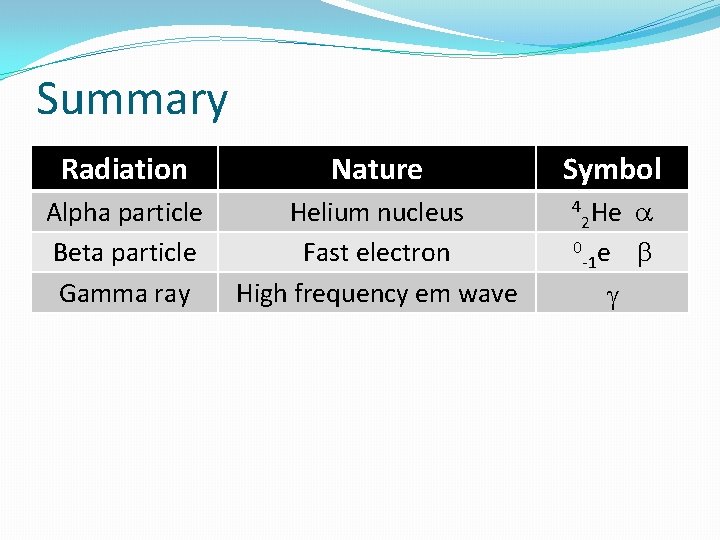
Summary Radiation Nature Alpha particle Beta particle Gamma ray Helium nucleus Fast electron High frequency em wave Symbol 2 He 0 e -1 4 g a b

�At Higher level we must be able to use equations to represent alpha and beta decay. �In the following equations both mass number and atomic number are conserved.

Alpha Decay �When a nucleus undergoes alpha decay the mass number decreases by 4 and the atomic number decreases by 2. �General equation: A ZX A-4 Z-2 Y 4 2 + He

Example �Iridium-168 undergoes alpha decay. Write the decay equation that shows this process. Solution 168 77 Ir 164 75 4 2 Re + He

Beta Decay �When a nucleus undergoes beta decay the mass number is unchanged and the atomic number increases by 1. �General equation: A ZX A Z +1 Y + 0 -1 e

Example �Calcium-46 undergoes beta decay. Write the decay equation that shows this process. Solution 46 20 Ca 46 21 Sc + 0 -1 e

Qs 43 to 45, page 19.