25 17 Carbohydrate Structure Determination Carbohydrate Structure Determination

25. 17 Carbohydrate Structure Determination

Carbohydrate Structure Determination Spectroscopy X-Ray Crystallography Chemical Tests once used extensively; now superceded by spectroscopic methods and x-ray crystallography reactions of carbohydrates can involve either open-chain form, furanose, or pyranose form

25. 18 Reduction of Carbohydrates
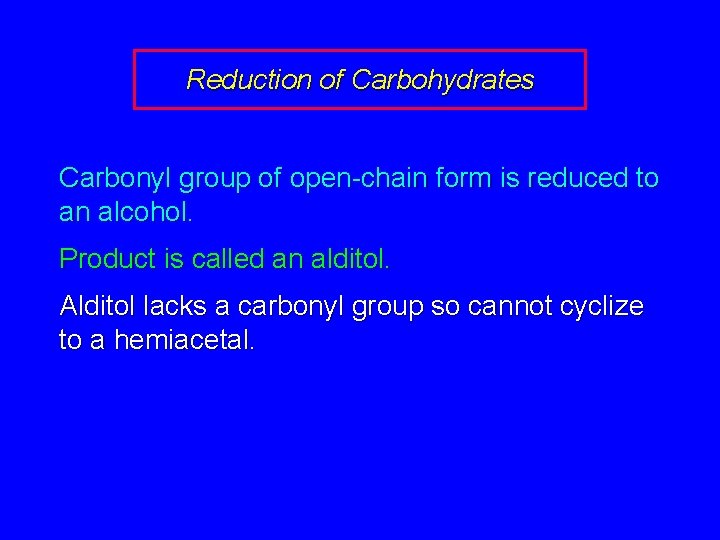
Reduction of Carbohydrates Carbonyl group of open-chain form is reduced to an alcohol. Product is called an alditol. Alditol lacks a carbonyl group so cannot cyclize to a hemiacetal.
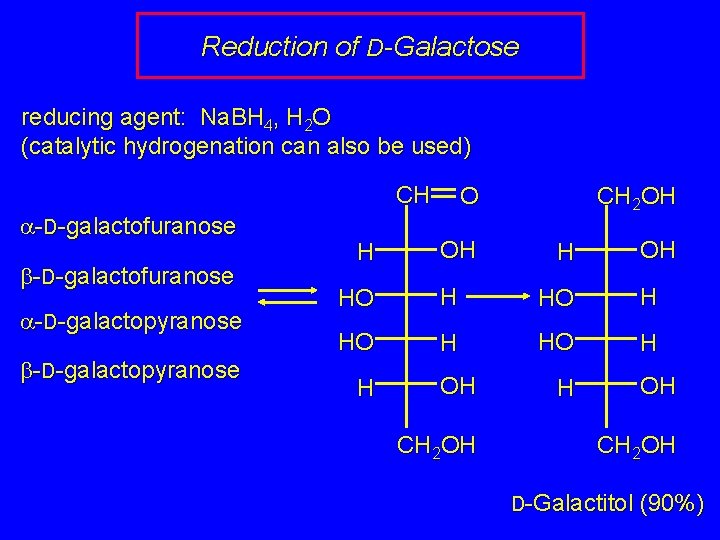
Reduction of D-Galactose reducing agent: Na. BH 4, H 2 O (catalytic hydrogenation can also be used) CH a-D-galactofuranose b-D-galactofuranose a-D-galactopyranose b-D-galactopyranose H CH 2 OH O OH HO H H OH CH 2 OH D-Galactitol (90%)

25. 19 Oxidation of Carbohydrates
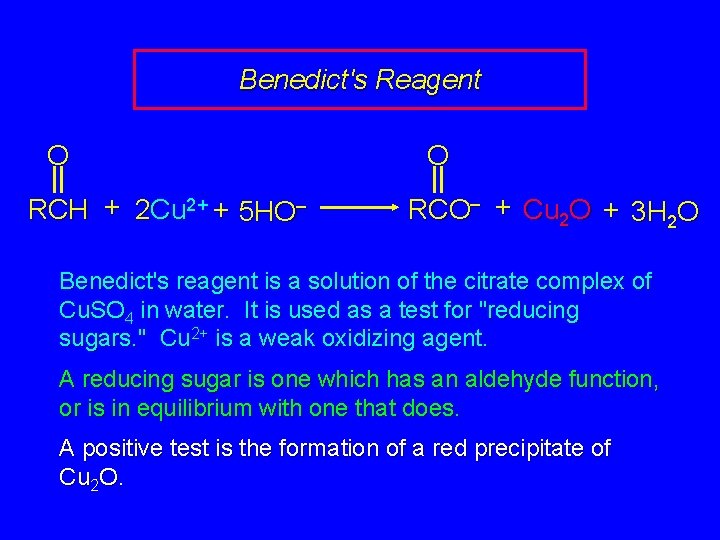
Benedict's Reagent O RCH + 2 Cu 2+ + 5 HO– O RCO– + Cu 2 O + 3 H 2 O Benedict's reagent is a solution of the citrate complex of Cu. SO 4 in water. It is used as a test for "reducing sugars. " Cu 2+ is a weak oxidizing agent. A reducing sugar is one which has an aldehyde function, or is in equilibrium with one that does. A positive test is the formation of a red precipitate of Cu 2 O.
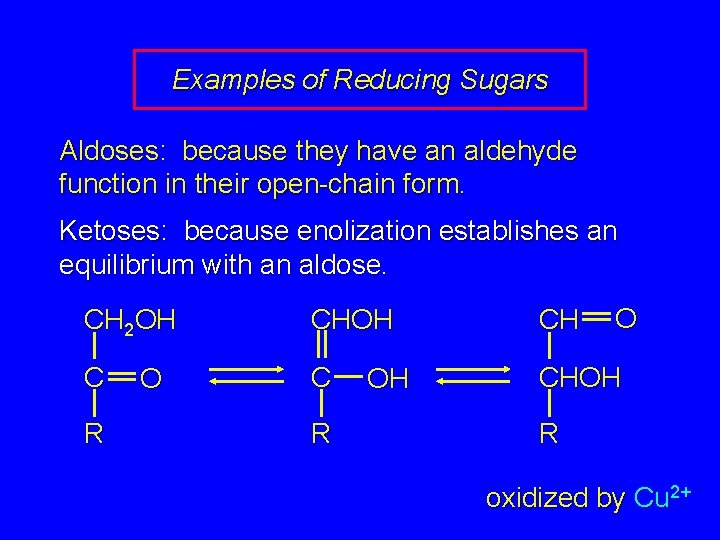
Examples of Reducing Sugars Aldoses: because they have an aldehyde function in their open-chain form. Ketoses: because enolization establishes an equilibrium with an aldose. O CH 2 OH CH C C CHOH R oxidized by Cu 2+
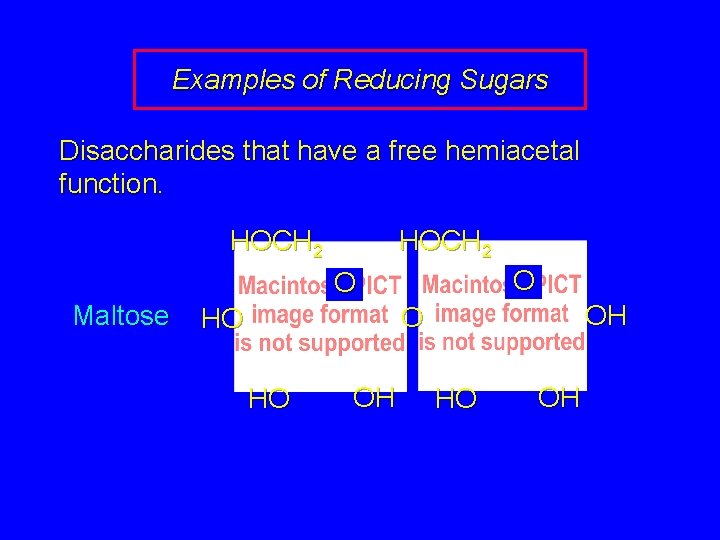
Examples of Reducing Sugars Disaccharides that have a free hemiacetal function. HOCH 2 O O Maltose OH O HO HO OH
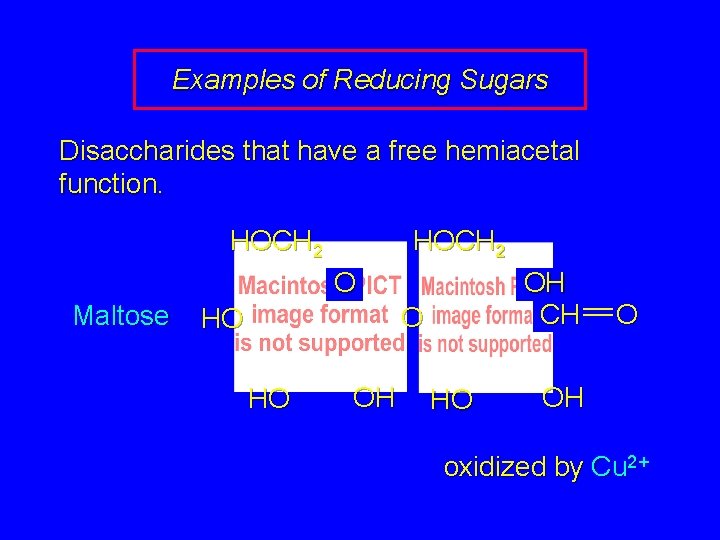
Examples of Reducing Sugars Disaccharides that have a free hemiacetal function. HOCH 2 O Maltose OH CH O HO HO OH HO O OH oxidized by Cu 2+

Glycosides are not reducing sugars HOCH 2 HO HO O OH OCH 3 Methyl a-D-glucopyranoside lacks a free hemiacetal function; cannot be in equilibrium with a species having an aldehyde function

Oxidation of Reducing Sugars The compounds formed on oxidation of reducing sugars are called aldonic acids. Aldonic acids exist as lactones when 5 - or 6 membered rings can form. A standard method for preparing aldonic acids uses Br 2 as the oxidizing agent.
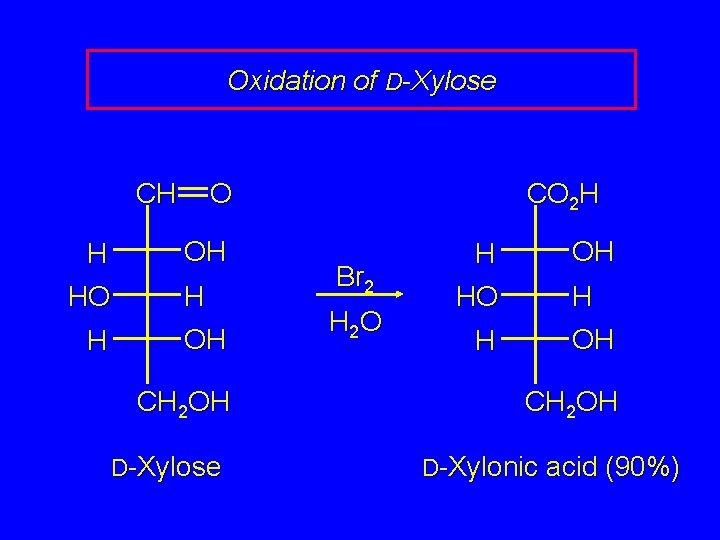
Oxidation of D-Xylose CH O H OH H CH 2 OH D-Xylose CO 2 H Br 2 H 2 O H OH H CH 2 OH D-Xylonic acid (90%)
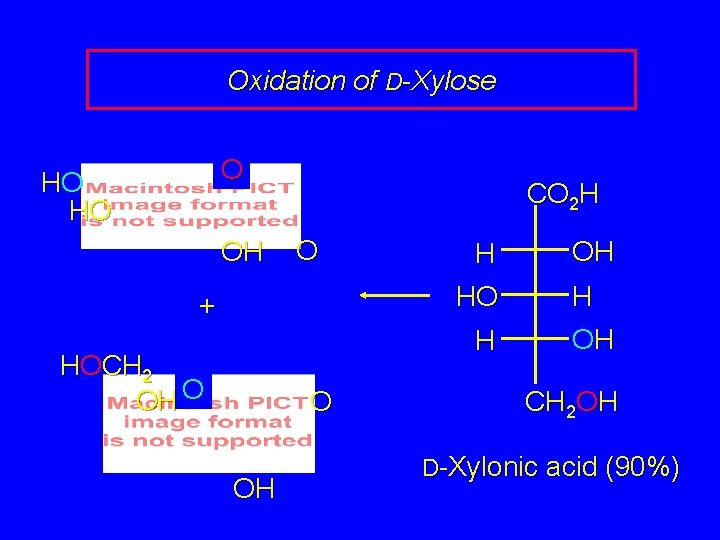
Oxidation of D-Xylose O HO HO OH CO 2 H O + H OH HO H OH H HOCH 2 OH O O OH CH 2 OH D-Xylonic acid (90%)

Nitric Acid Oxidation Nitric acid oxidizes both the aldehyde function and the terminal CH 2 OH of an aldose to CO 2 H. The products of such oxidations are called aldaric acids.
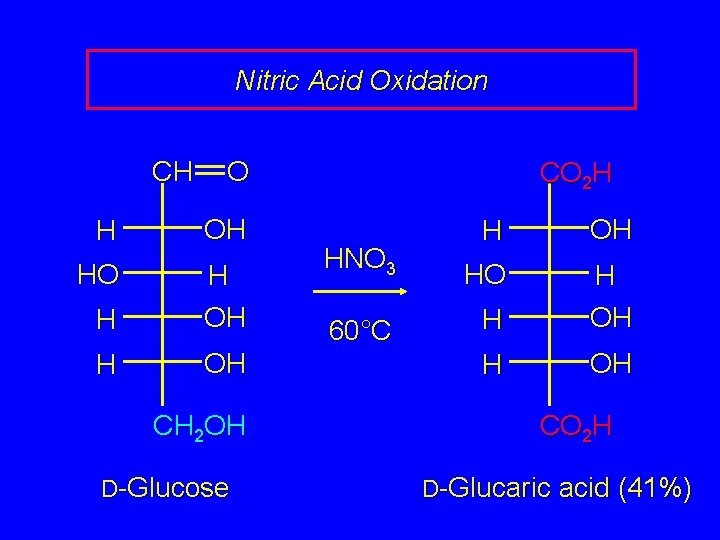
Nitric Acid Oxidation CH O H OH HO H H OH CH 2 OH D-Glucose CO 2 H HNO 3 H OH HO 60°C H H OH CO 2 H D-Glucaric acid (41%)

Uronic Acids Uronic acids contain both an aldehyde and a terminal CO 2 H function. CH O H OH HO H H OH CO 2 H HO 2 C HO HO O OH OH D-Glucuronic acid

25. 20 Cyanohydrin Formation and Carbohydrate Chain Extension

Extending the Carbohydrate Chain Carbohydrate chains can be extended by using cyanohydrin formation as the key step in C—C bond-making. The classical version of this method is called the Kiliani-Fischer synthesis. The following example is a more modern modification.
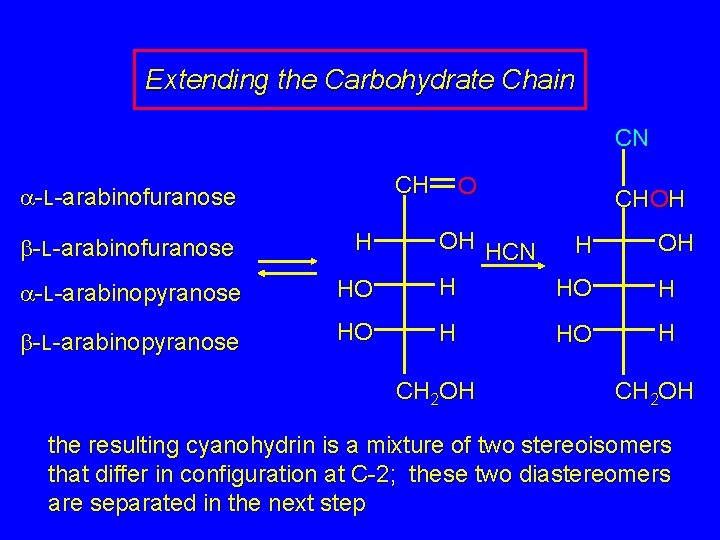
Extending the Carbohydrate Chain CN CH a-L-arabinofuranose O CHOH a-L-arabinopyranose HO OH HCN H H HO b-L-arabinopyranose HO H b-L-arabinofuranose H CH 2 OH HO OH H H CH 2 OH the resulting cyanohydrin is a mixture of two stereoisomers that differ in configuration at C-2; these two diastereomers are separated in the next step

Extending the Carbohydrate Chain CN CN CN H OH HO + H CHOH H OH separate H OH HO H HO H CH 2 OH L-Mannononitrile CH 2 OH L-Gluconononitrile CH 2 OH
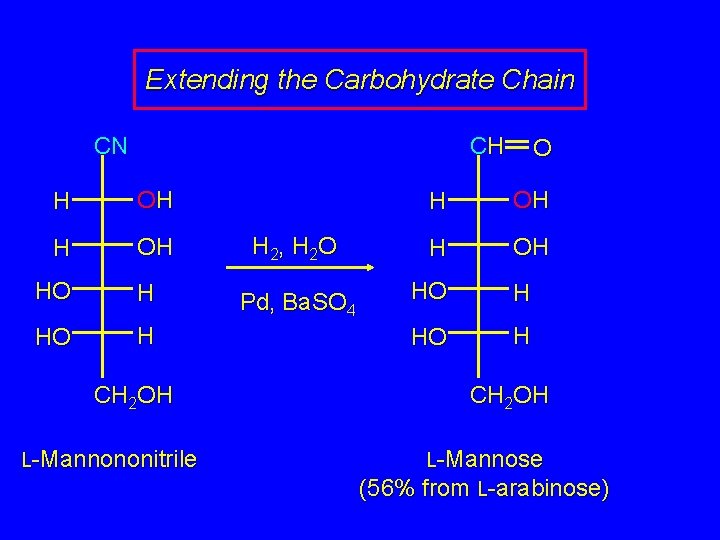
Extending the Carbohydrate Chain CN CH H OH HO H CH 2 OH L-Mannononitrile H 2, H 2 O Pd, Ba. SO 4 O H OH HO H CH 2 OH L-Mannose (56% from L-arabinose)
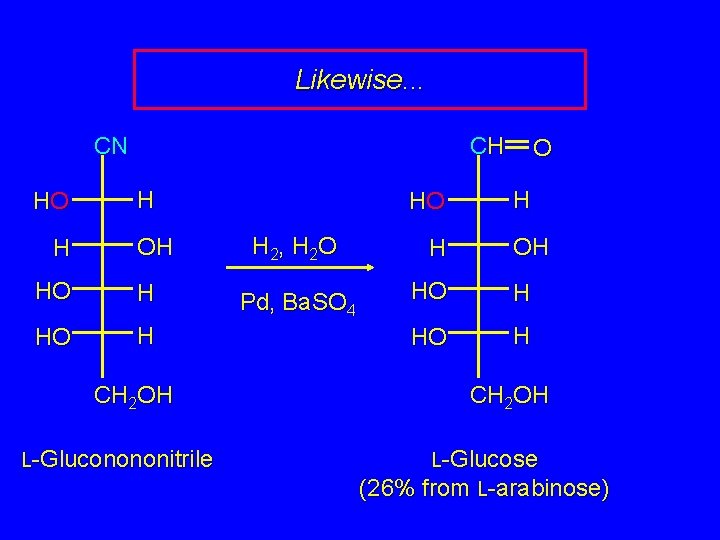
Likewise. . . CN HO H CH H OH HO H CH 2 OH L-Gluconononitrile HO H 2, H 2 O Pd, Ba. SO 4 H OH HO H CH 2 OH L-Glucose (26% from L-arabinose)

25. 21 Epimerization, Isomerization, and Retro-Aldol Reactions of Carbohydrates

Enol Forms of Carbohydrates Enolization of an aldose scrambles the stereochemistry at C-2. This process is called epimerization. Diastereomers that differ in stereochemistry at only one of their stereogenic centers are called epimers. D-Glucose and D-mannose, for example, are epimers.
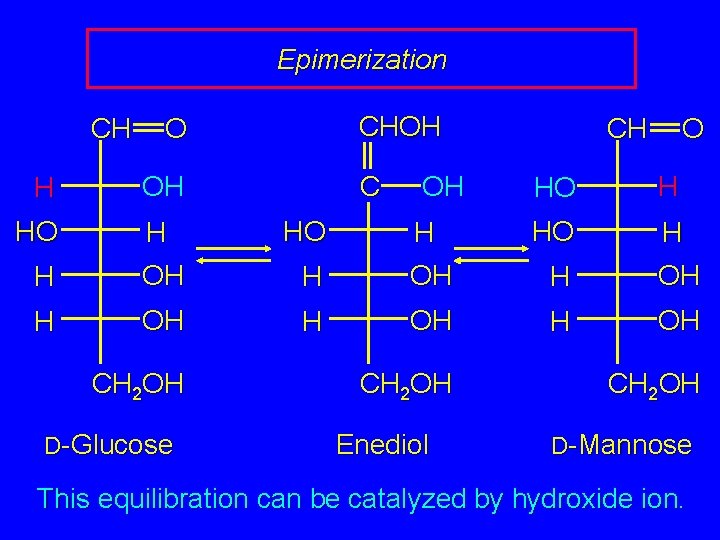
Epimerization CH CHOH O C H HO H H OH OH HO HO H H OH CH 2 OH D-Glucose O HO H OH CH CH 2 OH Enediol CH 2 OH D-Mannose This equilibration can be catalyzed by hydroxide ion.

Enol Forms of Carbohydrates The enediol intermediate on the preceding slide can undergo a second reaction. It can lead to the conversion of D-glucose or D-mannose (aldoses) to D-fructose (ketose).
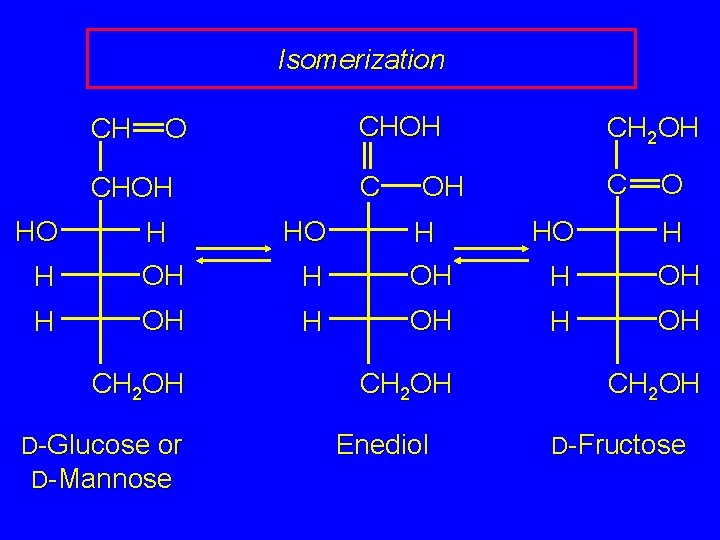
Isomerization CH O CHOH HO HO H H OH CH 2 OH D-Glucose or D-Mannose CHOH CH 2 OH C C OH O HO H H OH CH 2 OH Enediol CH 2 OH D-Fructose

Retro-Aldol Cleavage The D-fructose 6 -phosphate formed according to the preceding slide undergoes phosphorylation of its free CH 2 OH group to give D-fructose 1, 6 diphosphate. D-Fructose 1, 6 -diphosphate is cleaved to two 3 - carbon products by a reverse aldol reaction. This retro-aldol cleavage is catalyzed by the enzyme aldolase.
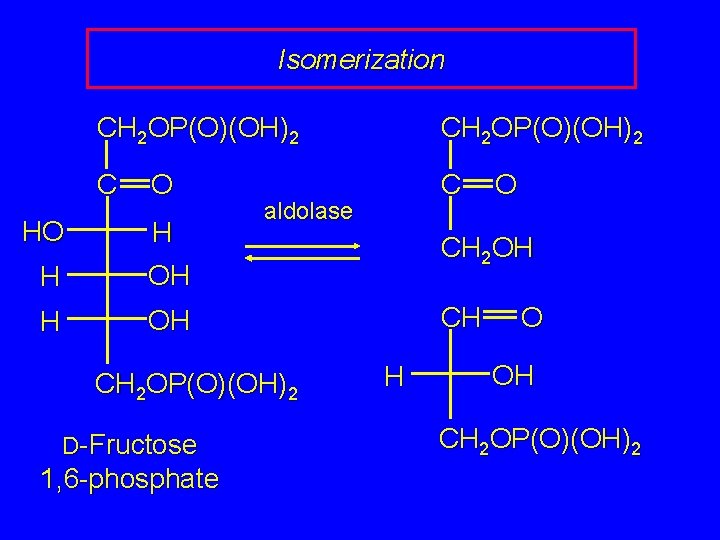
Isomerization HO CH 2 OP(O)(OH)2 C C O H H OH aldolase CH 2 OP(O)(OH)2 D-Fructose 1, 6 -phosphate O CH 2 OH CH H O OH CH 2 OP(O)(OH)2

25. 22 Acylation and Alkylation of Hydroxyl Groups in Carbohydrates

Reactivity of Hydroxyl Groups in Carbohydrates Hydroxyl groups in carbohydrates undergo reactions typical of alcohols. acylation alkylation
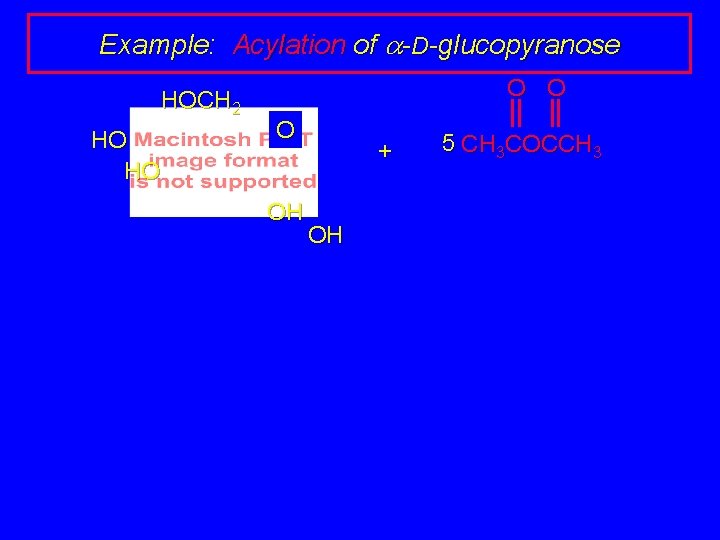
Example: Acylation of a-D-glucopyranose HOCH 2 HO HO O OH + OH 5 CH 3 COCCH 3
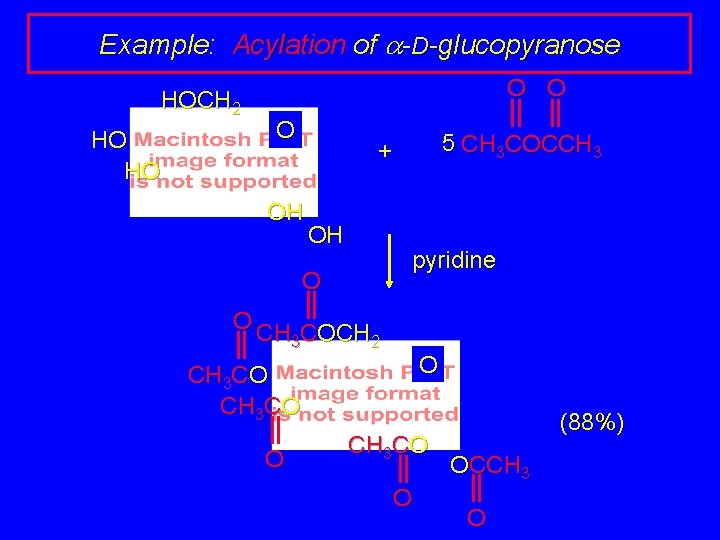
Example: Acylation of a-D-glucopyranose HOCH 2 HO HO O 5 CH 3 COCCH 3 + OH OH pyridine O O CH 3 COCH 2 O CH 3 CO O (88%) OCCH 3 O
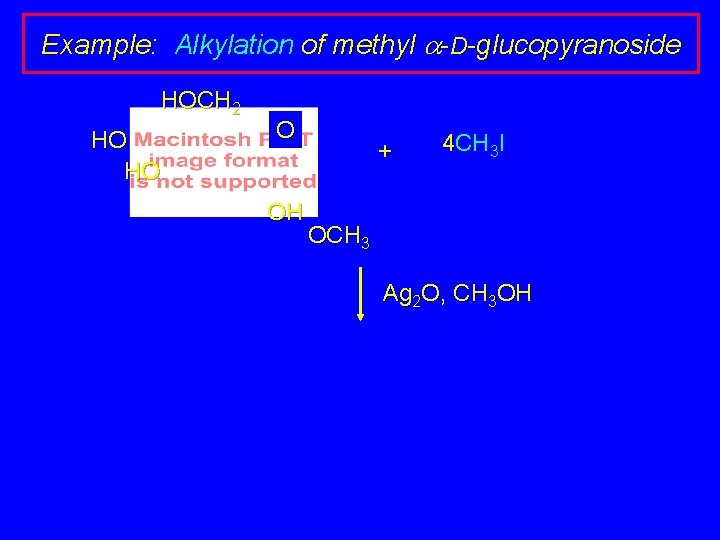
Example: Alkylation of methyl a-D-glucopyranoside HOCH 2 HO HO O OH + 4 CH 3 I OCH 3 Ag 2 O, CH 3 OH
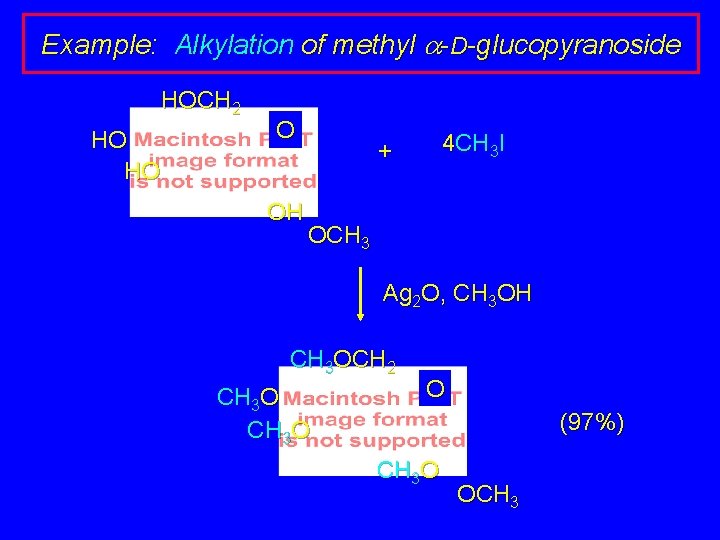
Example: Alkylation of methyl a-D-glucopyranoside HOCH 2 HO HO O OH 4 CH 3 I + OCH 3 Ag 2 O, CH 3 OH CH 3 OCH 2 CH 3 O O (97%) CH 3 O OCH 3
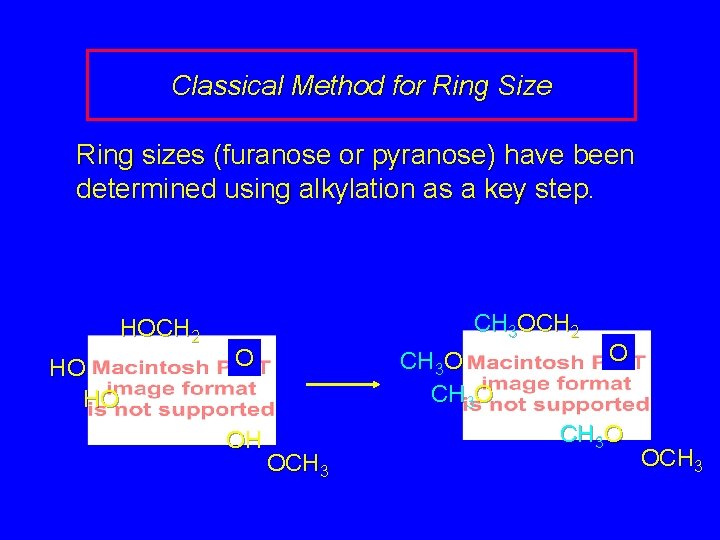
Classical Method for Ring Size Ring sizes (furanose or pyranose) have been determined using alkylation as a key step. HOCH 2 HO HO CH 3 OCH 2 O OH CH 3 O OCH 3 O CH 3 O OCH 3
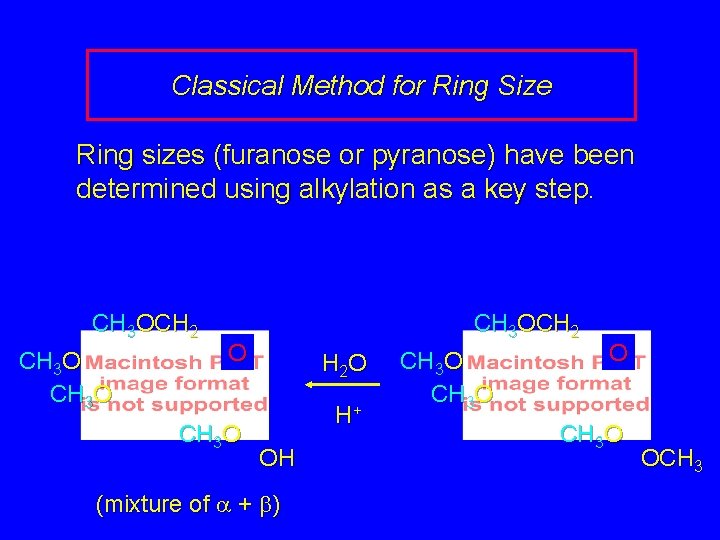
Classical Method for Ring Size Ring sizes (furanose or pyranose) have been determined using alkylation as a key step. CH 3 OCH 2 CH 3 OCH 2 O CH 3 O H 2 O H+ OH (mixture of a + b) CH 3 O OCH 3
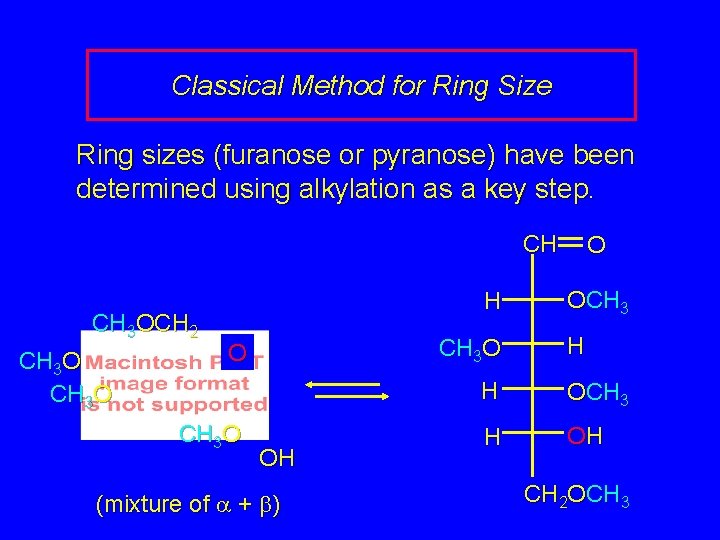
Classical Method for Ring Size Ring sizes (furanose or pyranose) have been determined using alkylation as a key step. CH CH 3 OCH 2 CH 3 O H CH 3 O OH (mixture of a + b) O OCH 3 H H OCH 3 H OH CH 2 OCH 3
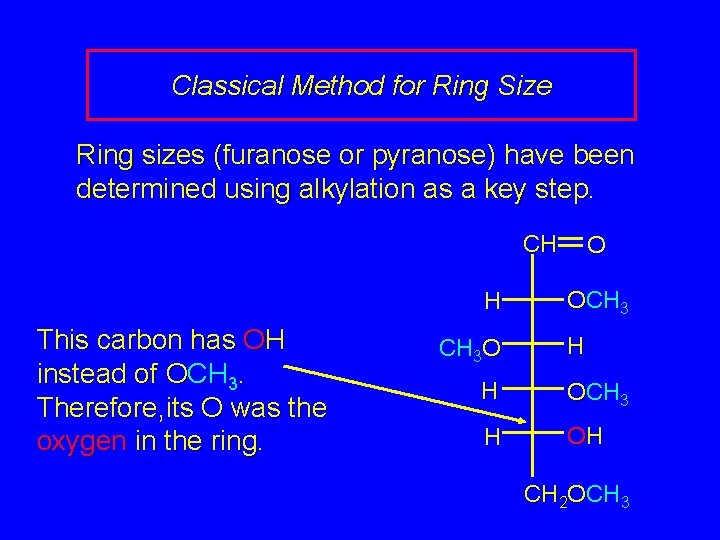
Classical Method for Ring Size Ring sizes (furanose or pyranose) have been determined using alkylation as a key step. CH H This carbon has OH instead of OCH 3. Therefore, its O was the oxygen in the ring. CH 3 O O OCH 3 H H OCH 3 H OH CH 2 OCH 3

25. 23 Periodic Acid Oxidation of Carbohydrates
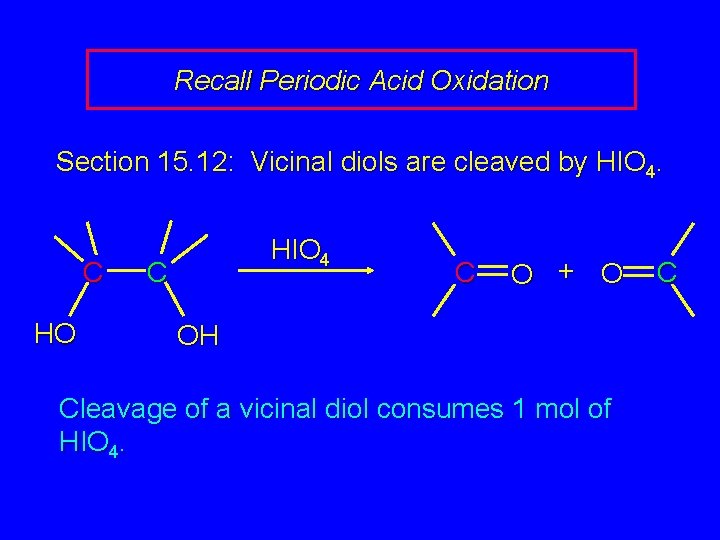
Recall Periodic Acid Oxidation Section 15. 12: Vicinal diols are cleaved by HIO 4. C HO HIO 4 C C O + O OH Cleavage of a vicinal diol consumes 1 mol of HIO 4. C

Also Cleaved by HIO 4 a-Hydroxy carbonyl compounds R O RC HIO 4 C OH C O + O C HO Cleavage of an a-hydroxy carbonyl compound consumes 1 mol of HIO 4. One of the products is a carboxylic acid.

Also Cleaved by HIO 4 R 2 C HO Compounds that contain three contiguous carbons bearing OH groups O CH CR'2 HIO 4 R 2 C O + HCOH OH OH + R'2 C O 2 mol of HIO 4 are consumed. 1 mole of formic acid is produced.
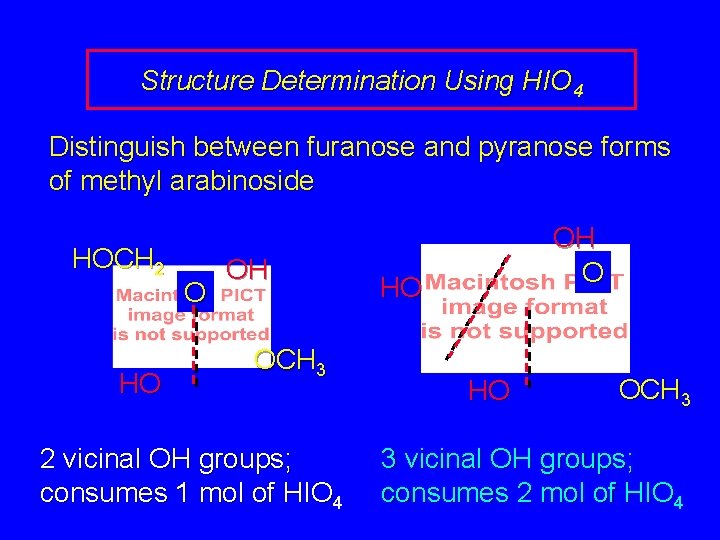
Structure Determination Using HIO 4 Distinguish between furanose and pyranose forms of methyl arabinoside HOCH 2 HO O OH OCH 3 2 vicinal OH groups; consumes 1 mol of HIO 4 OH O HO HO OCH 3 3 vicinal OH groups; consumes 2 mol of HIO 4
- Slides: 45