23 8 The EliminationAddition Mechanism of Nucleophilic Aromatic





- Slides: 19

23. 8 The Elimination-Addition Mechanism of Nucleophilic Aromatic Substitution: Benzyne

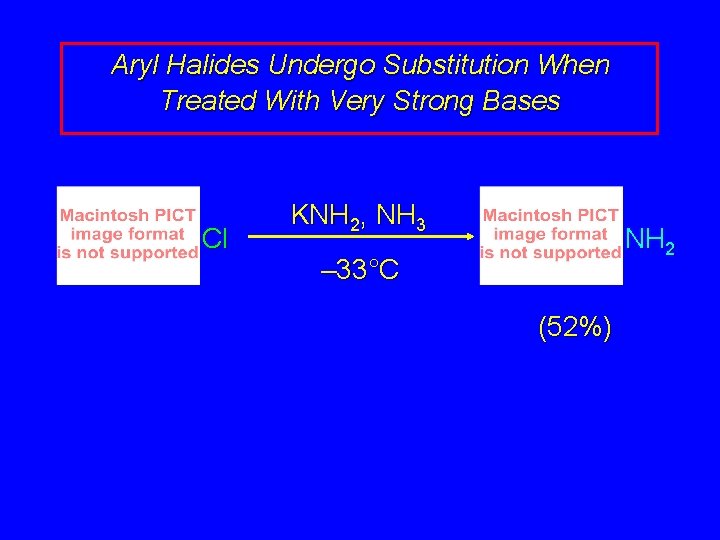
Aryl Halides Undergo Substitution When Treated With Very Strong Bases Cl KNH 2, NH 3 NH 2 – 33°C (52%)

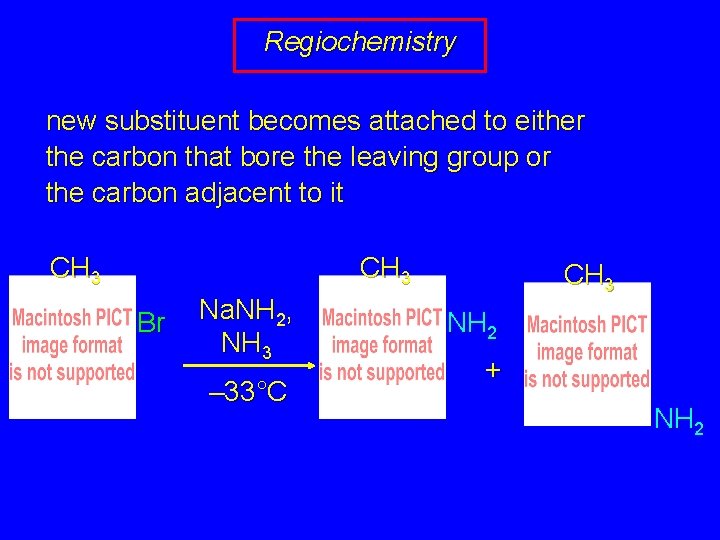
Regiochemistry new substituent becomes attached to either the carbon that bore the leaving group or the carbon adjacent to it CH 3 Br Na. NH 2, NH 3 – 33°C CH 3 NH 2 + NH 2

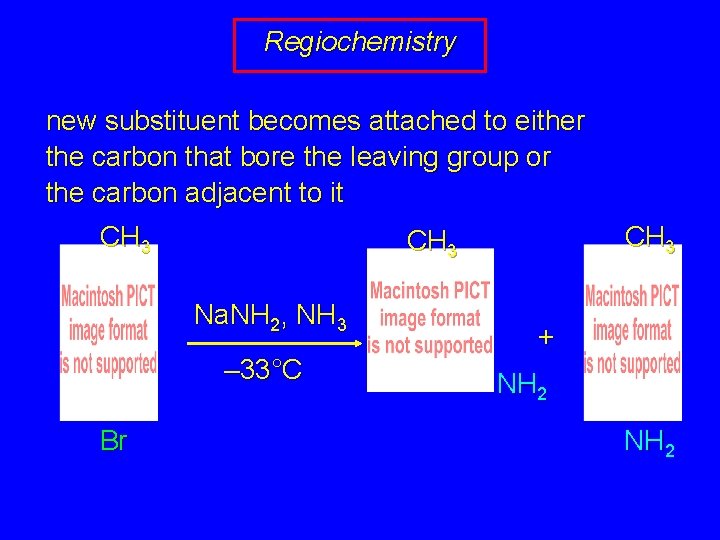
Regiochemistry new substituent becomes attached to either the carbon that bore the leaving group or the carbon adjacent to it CH 3 Na. NH 2, NH 3 – 33°C Br CH 3 + NH 2

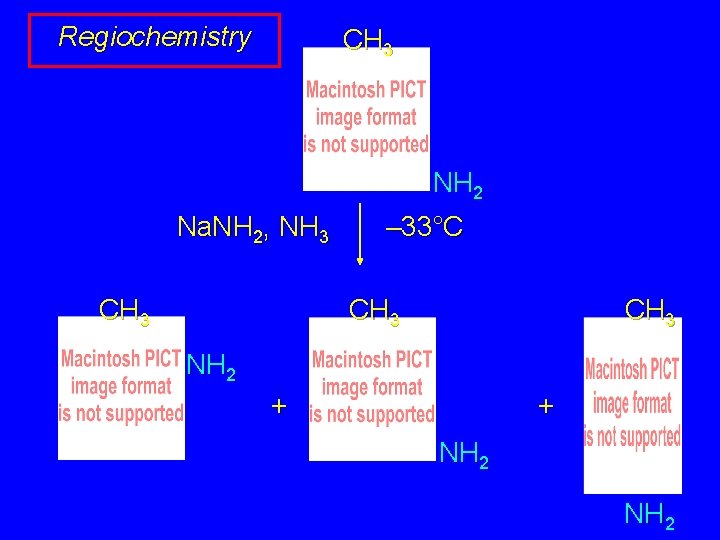
Regiochemistry CH 3 NH 2 Na. NH 2, NH 3 CH 3 – 33°C CH 3 NH 2 + + NH 2

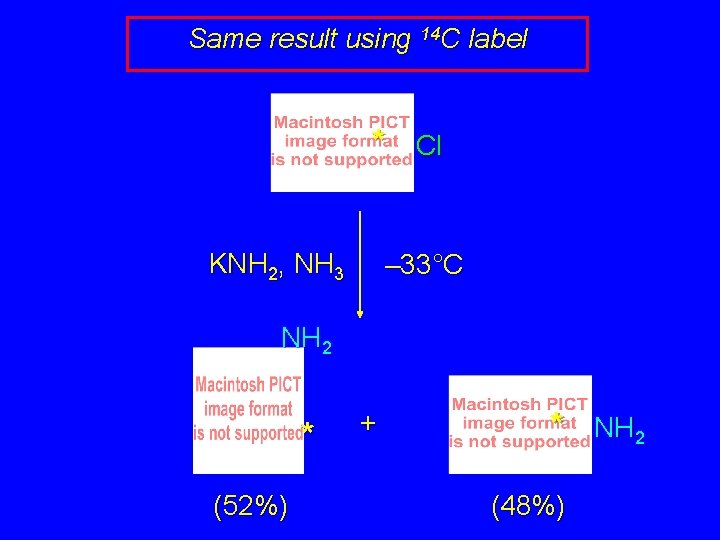
Same result using 14 C label * KNH 2, NH 3 Cl – 33°C NH 2 * (52%) + * (48%) NH 2

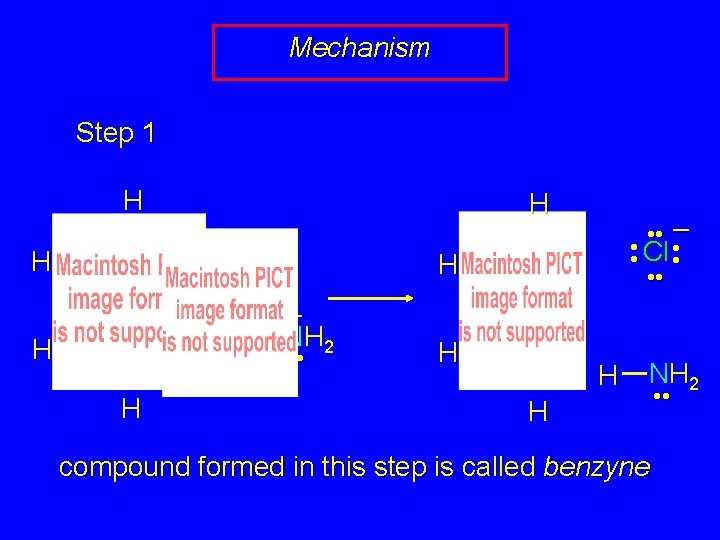
Mechanism Step 1 H • • Cl • • H H H – • • NH 2 • •

Mechanism Step 1 H H • • Cl • • H H H • • – • • Cl • • • • – • • NH 2 • • H H NH 2 H compound formed in this step is called benzyne • •

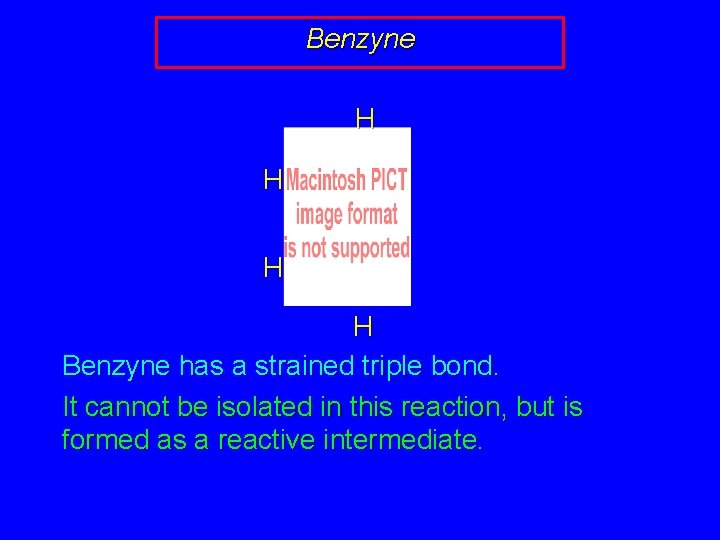
Benzyne H H Benzyne has a strained triple bond. It cannot be isolated in this reaction, but is formed as a reactive intermediate.

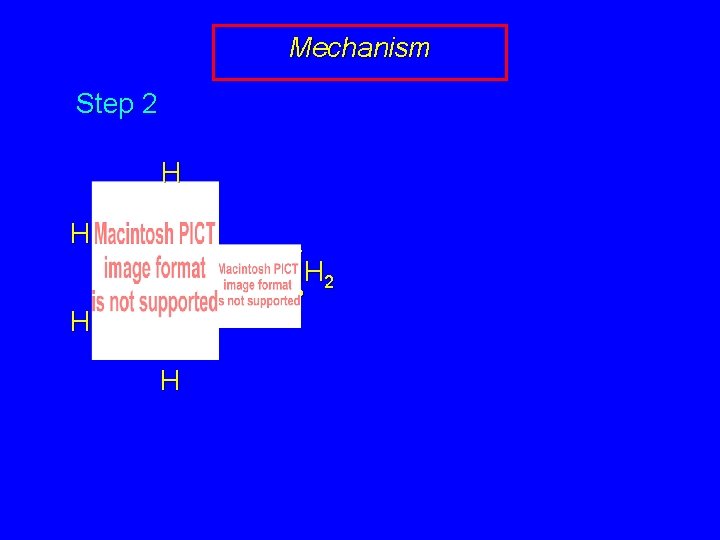
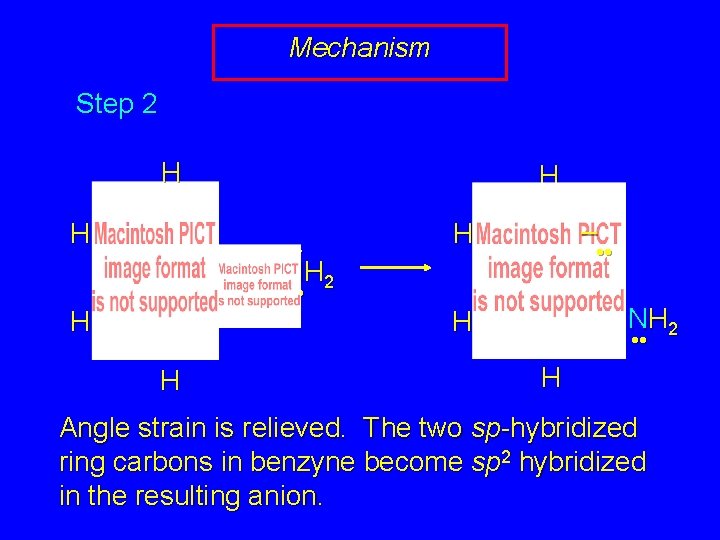
Mechanism Step 2 H H – • • NH 2 • • H H

Mechanism Step 2 H H H – • • NH 2 • • H H – H • • NH 2 H • • H Angle strain is relieved. The two sp-hybridized ring carbons in benzyne become sp 2 hybridized in the resulting anion.

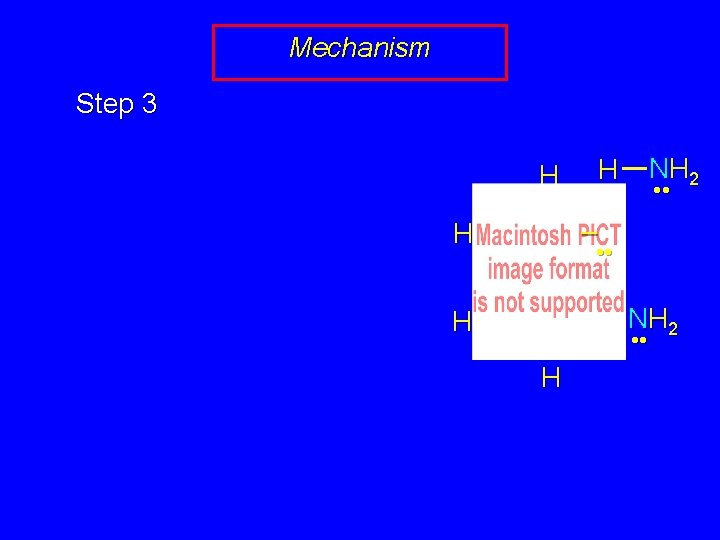
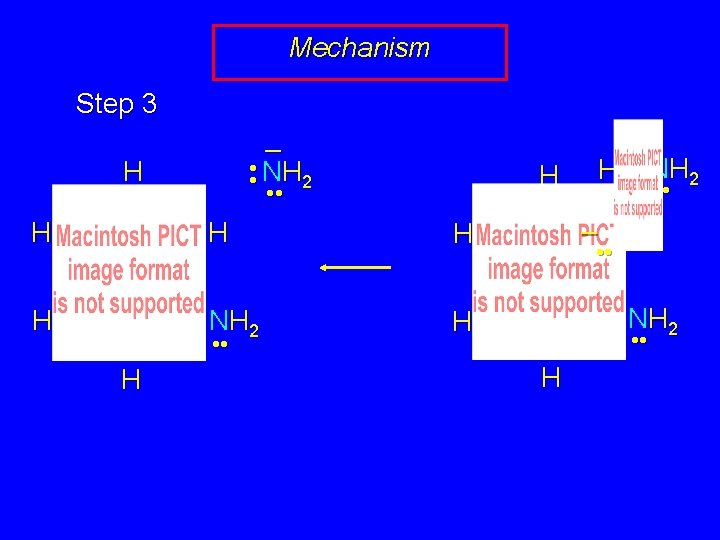
Mechanism Step 3 NH 2 H H • • – H • • NH 2 H • • H


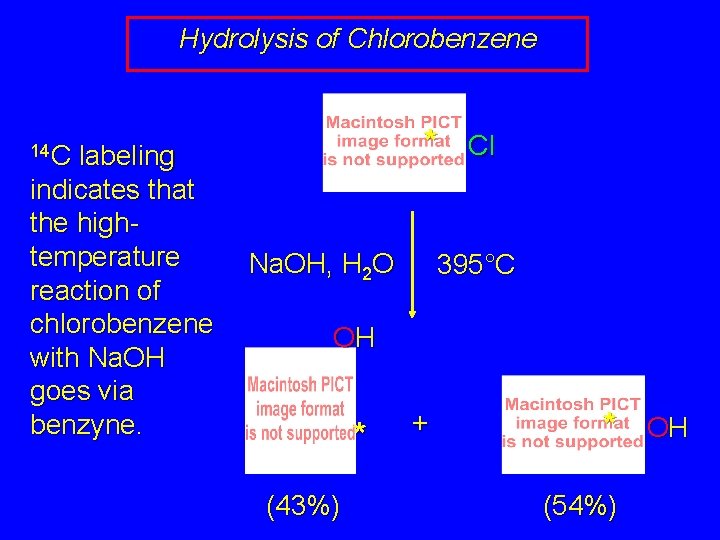
Hydrolysis of Chlorobenzene labeling indicates that the hightemperature reaction of chlorobenzene with Na. OH goes via benzyne. * 14 C Na. OH, H 2 O Cl 395°C OH * (43%) + * (54%) OH

23. 9 Diels-Alder Reactions of Benzyne

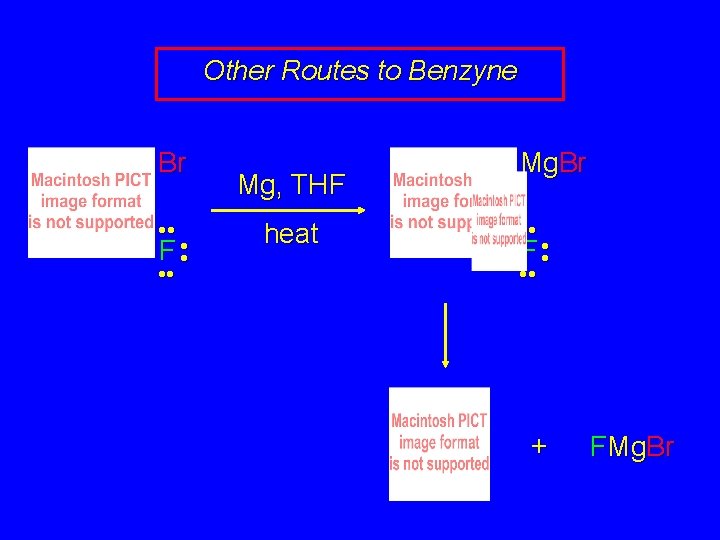
Other Routes to Benzyne can be prepared as a reactive intermediate by methods other than treatment of chlorobenzene with strong bases. Another method involves loss of fluoride ion from the Grignard reagent of 1 -bromo-2 fluorobenzene.

Other Routes to Benzyne Br • • F • • Mg, THF heat Mg. Br • • F • • + FMg. Br

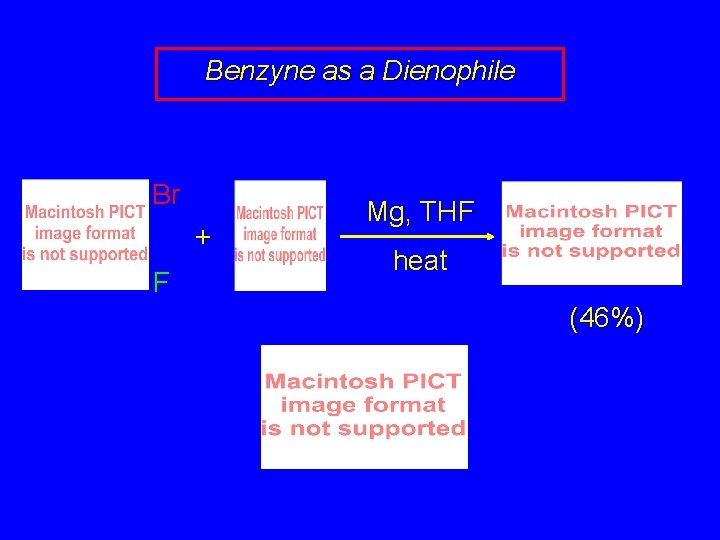
Benzyne as a Dienophile Benzyne is a fairly reactive dienophile, and gives Diels-Alder adducts when generated in the presence of conjugated dienes.

Benzyne as a Dienophile Br + F Mg, THF heat (46%)