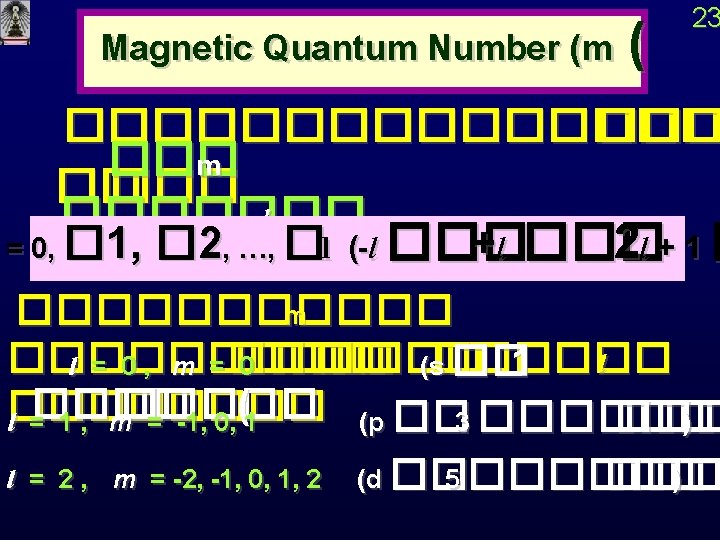
22 Orbital Quantum Number Angular Momentum Quantum Number


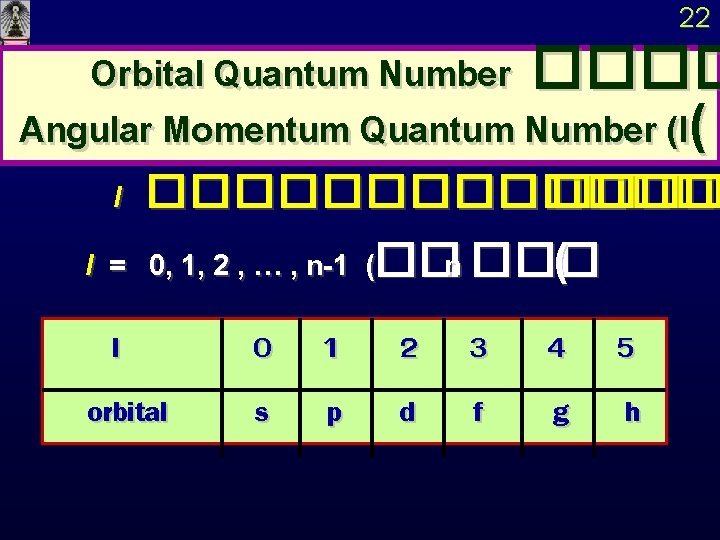
22 Orbital Quantum Number ���� Angular Momentum Quantum Number (l( l ������� l = 0, 1, 2 , … , n-1 (��n ��� ( l orbital 0 s 1 p 2 d 3 f 4 g 5 h
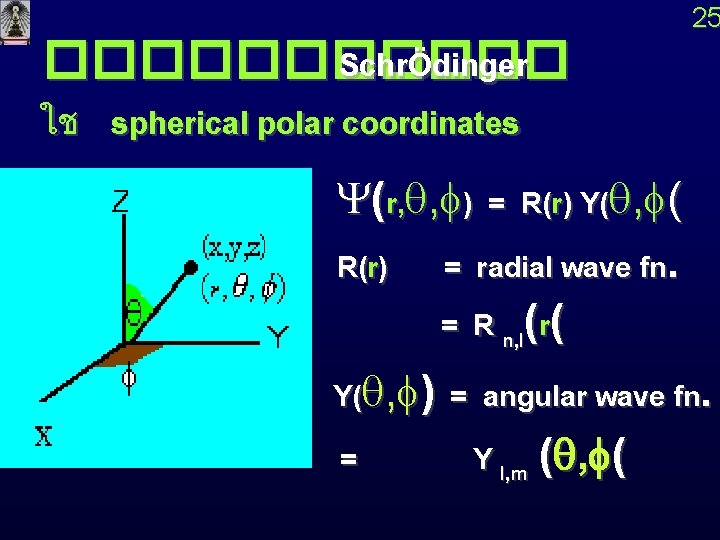
25 ������ SchrÖdinger ใช spherical polar coordinates Y(r, q, f) = R(r) Y(q, f( R(r) = radial wave fn. = R n, l(r( Y(q, f) = angular wave fn. = Y l, m (q, f(

27 ���� �� ����� (Atomic Orbitals 1 s orbital n = 1, l = 0 , m = 0 Y(r, q, f) = R(r) Y(q, f kr radial wave function R 1, 0(r) = e
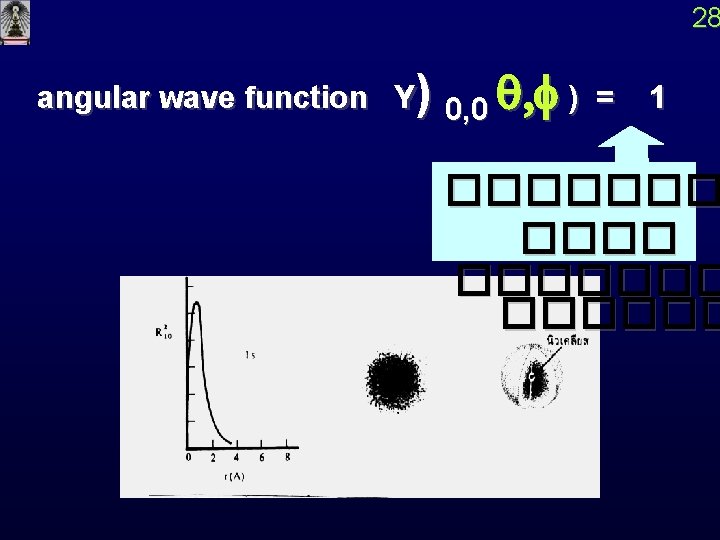
28 angular wave function Y) 0, 0 q, f ) = 1 �������
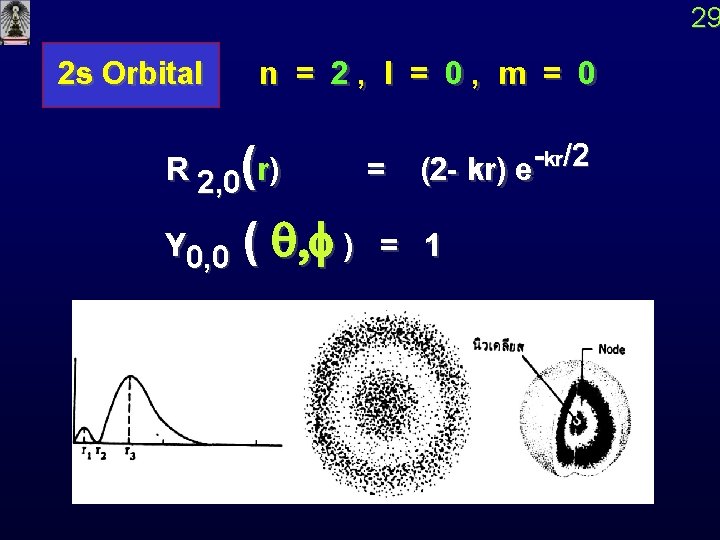
29 2 s Orbital n = 2, l = 0, m = 0 R 2, 0(r) = kr /2 (2 - kr) e Y 0, 0 ( q, f ) = 1
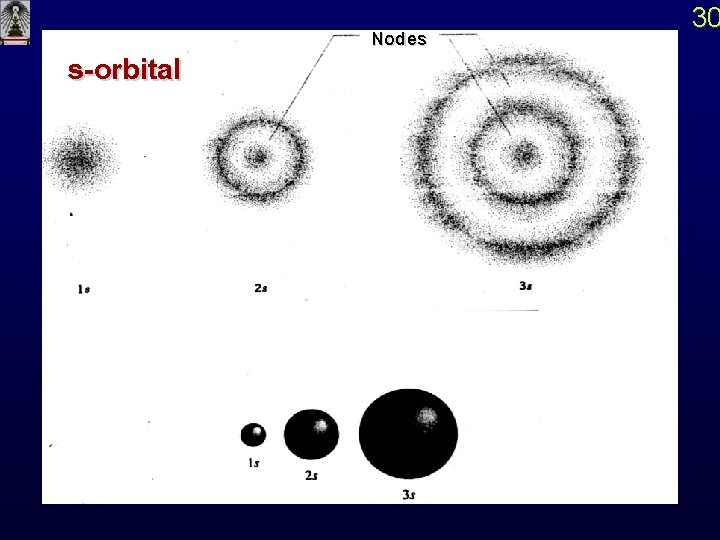
s-orbital Nodes 30
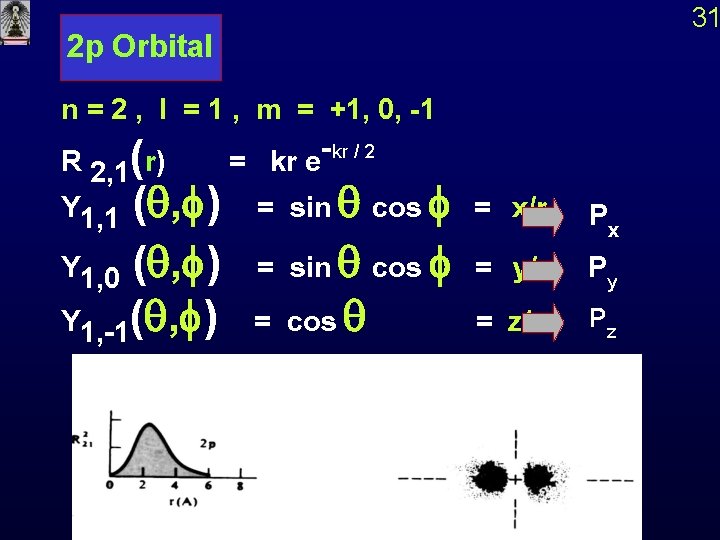
2 p Orbital n = 2 , l = 1 , m = +1, 0, -1 R 2, 1(r) = kr e-kr / 2 Y 1, 1 (q, f) = sin q cos f = x/r Px Y 1, 0 (q, f) = sin q cos f = y/r Py Y 1, -1(q, f) = cos q = z/r Pz 31
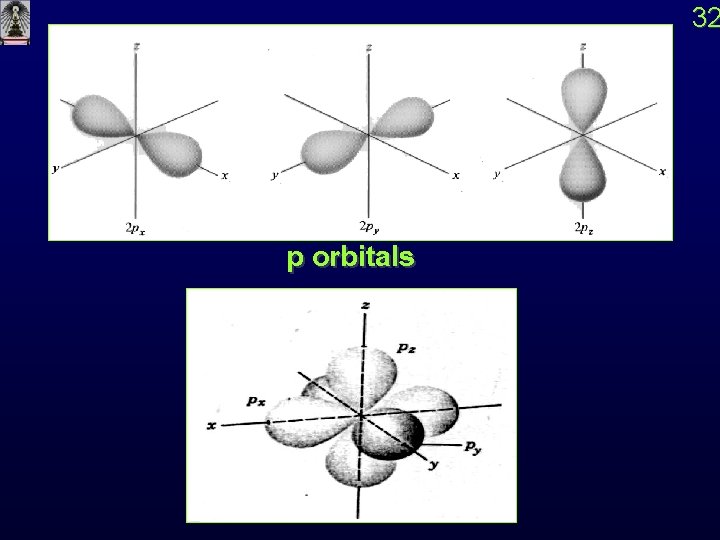
32 p orbitals
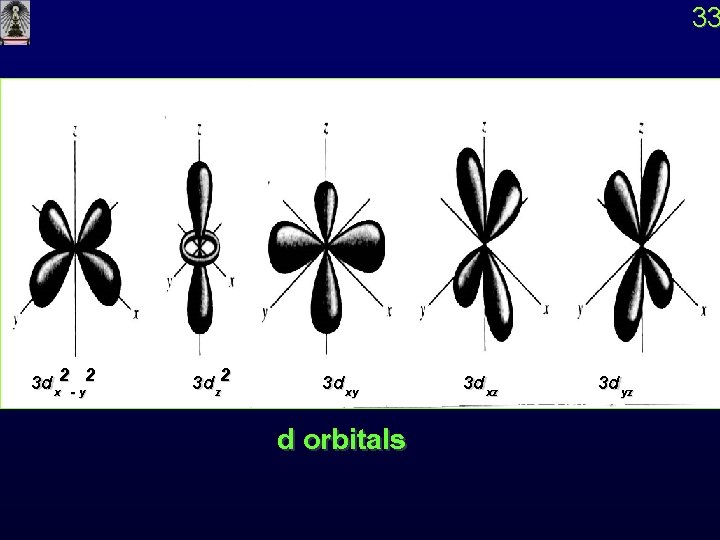
33 3 dx 2 - y 2 3 dz 2 3 dxy d orbitals 3 dxz 3 dyz
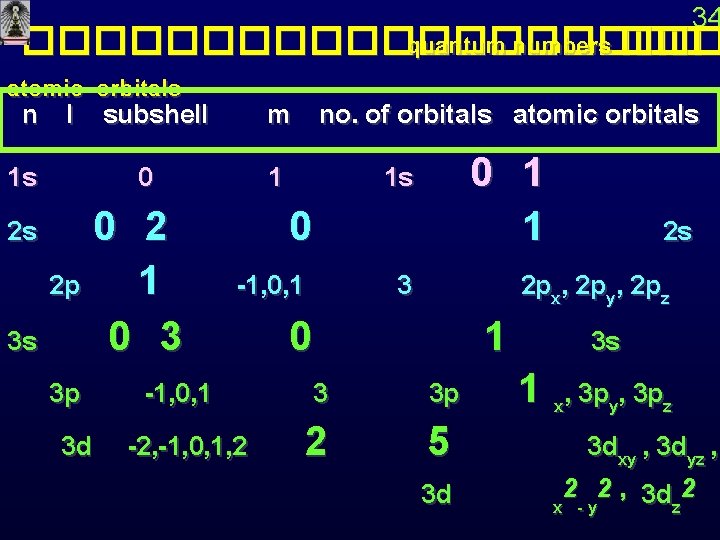
34 ���������� quantum numbers ��� atomic orbitals n l subshell 1 s 2 s m no. of orbitals atomic orbitals 0 1 0 2 p 1 -1, 0, 1 3 s 0 3 p -1, 0, 1 3 3 d -2, -1, 0, 1, 2 2 0 1 1 2 s 3 2 px, 2 py, 2 pz 1 3 s 3 p 1 x, 3 py, 3 pz 5 3 dxy , 3 dyz , 2 2 , 3 d 2 3 d x -y z 1 s
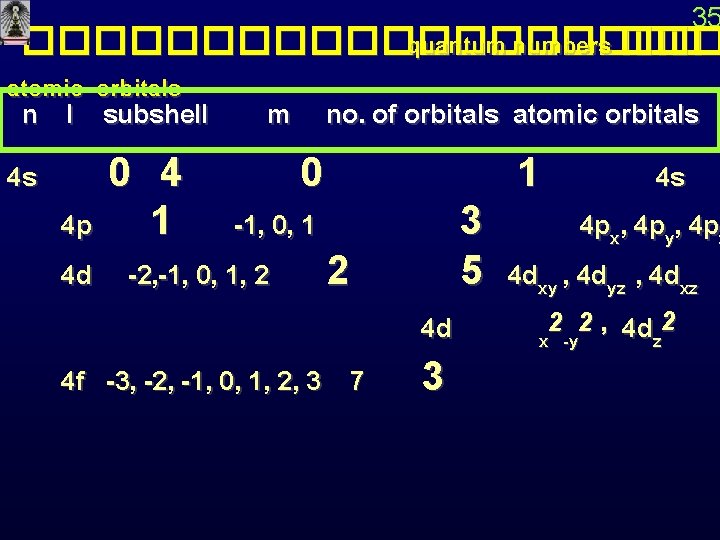
35 ���������� quantum numbers ��� atomic orbitals n l subshell 4 s m no. of orbitals atomic orbitals 0 4 p 1 -1, 0, 1 4 d -2, -1, 0, 1, 2 2 4 f -3, -2, -1, 0, 1, 2, 3 7 1 4 s 3 4 px, 4 py, 4 pz 5 4 dxy , 4 dyz , 4 dxz 2 2 , 4 d 2 4 d x -y z 3

37
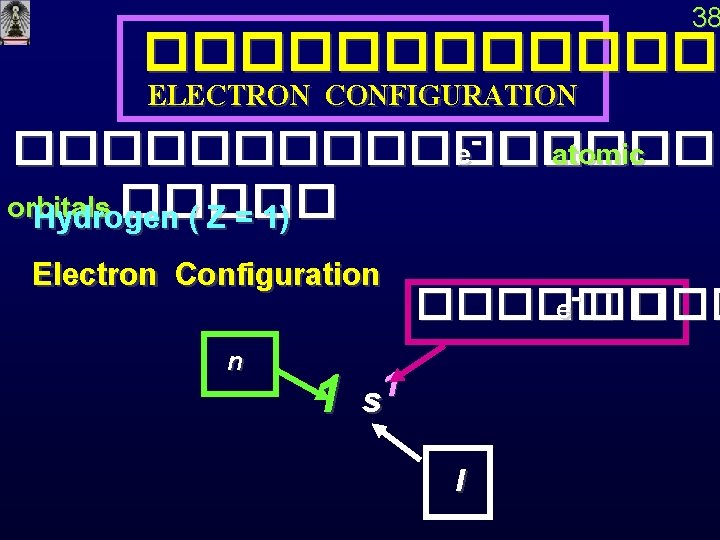
38 ������� ELECTRON CONFIGURATION ��������� e- �� atomic orbitals ����� Hydrogen ( Z = 1) Electron Configuration n 1 ����� e- �� � 1 s l
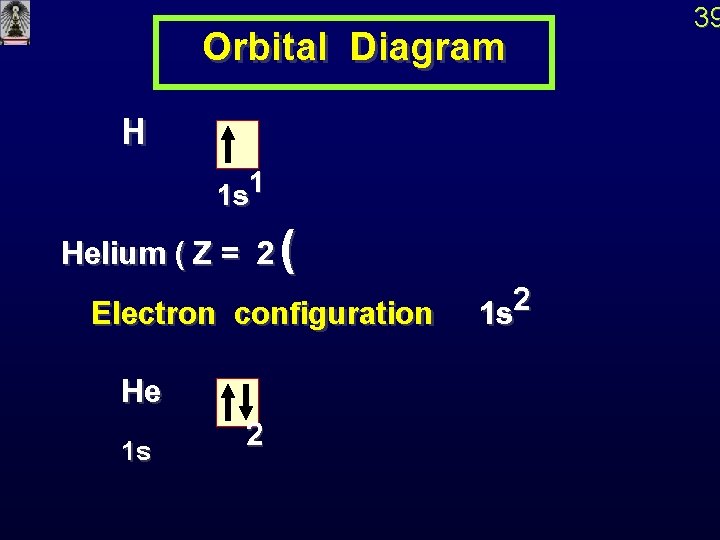
Orbital Diagram H 1 1 s Helium ( Z = 2 ( 2 Electron configuration 1 s He 1 s 2 39
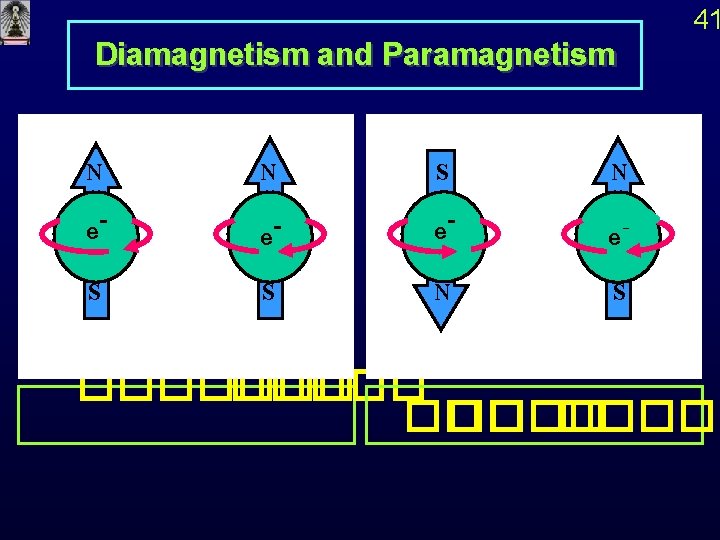
Diamagnetism and Paramagnetism N e-- S S N S 41 N ������� �� �����
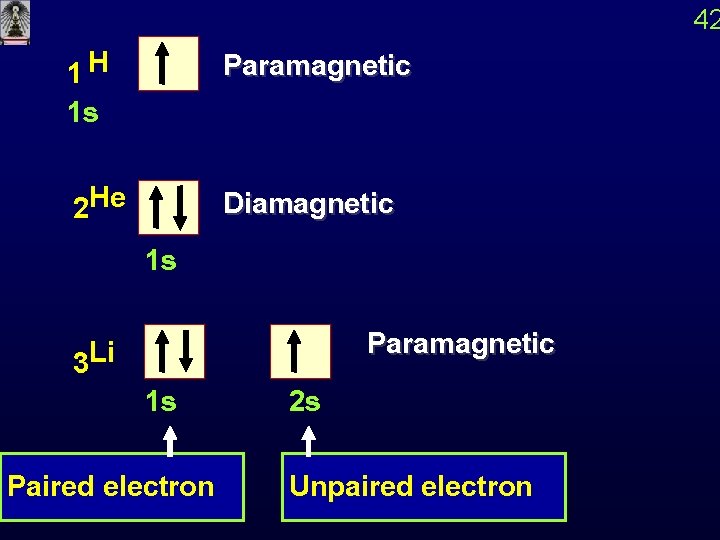
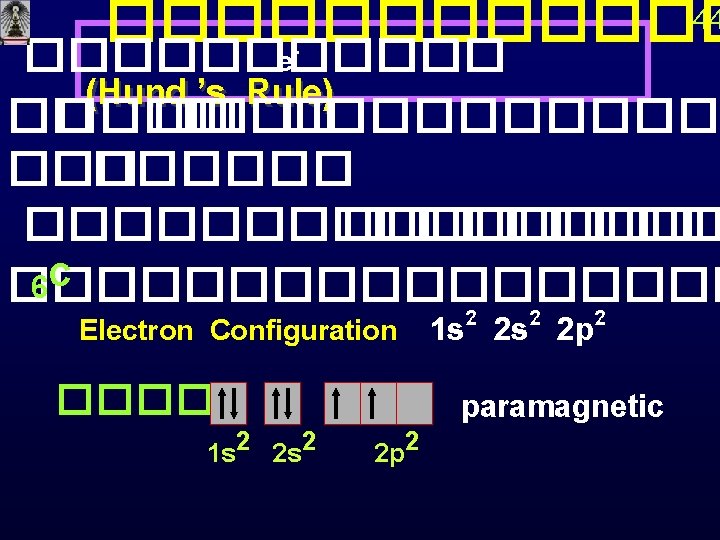
42 1 H Paramagnetic 2 He Diamagnetic 1 s 1 s 3 Li 1 s Paired electron 2 s Paramagnetic Unpaired electron
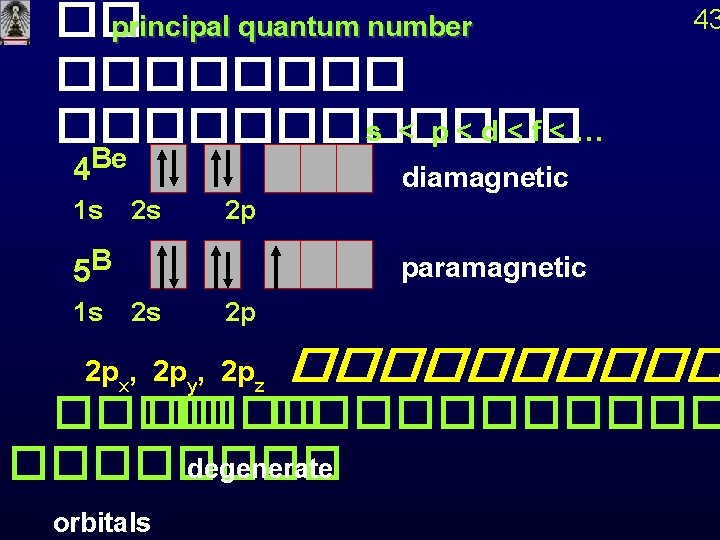
�� principal quantum number ������������ s < p<d<f<… 4 Be diamagnetic 1 s 2 s 5 B 1 s 2 s 43 2 p 2 p paramagnetic 2 px, 2 py, 2 pz ����� �� ������ degenerate orbitals
- Slides: 25