21 8 Barbiturates Barbituric acid is made from

21. 8 Barbiturates
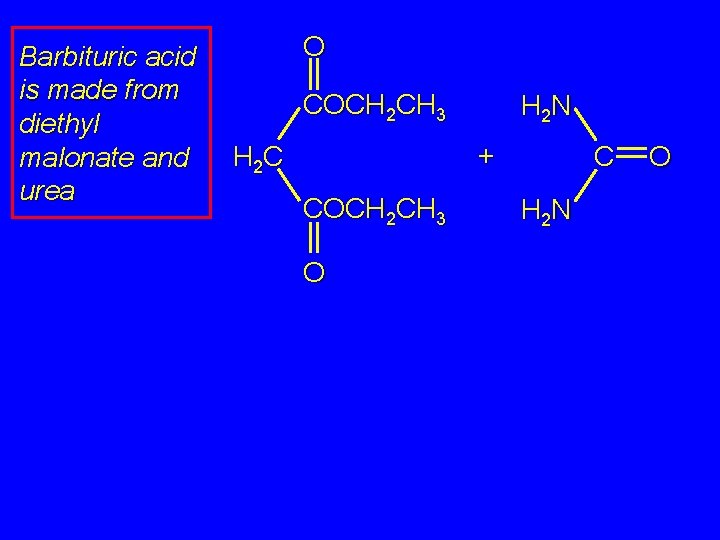
Barbituric acid is made from diethyl malonate and urea O COCH 2 CH 3 H 2 N + H 2 C COCH 2 CH 3 O C H 2 N O
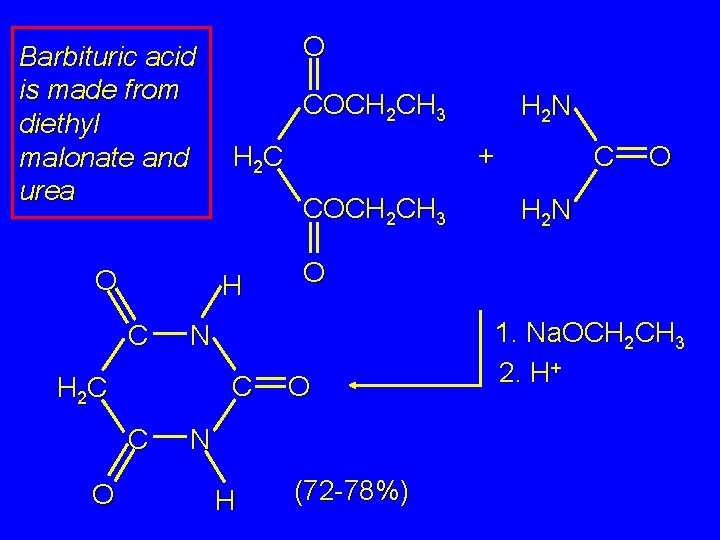
Barbituric acid is made from diethyl malonate and urea O COCH 2 CH 3 C C O N H C O H 2 N O N H 2 C H 2 N + H 2 C H C O O (72 -78%) 1. Na. OCH 2 CH 3 2. H+
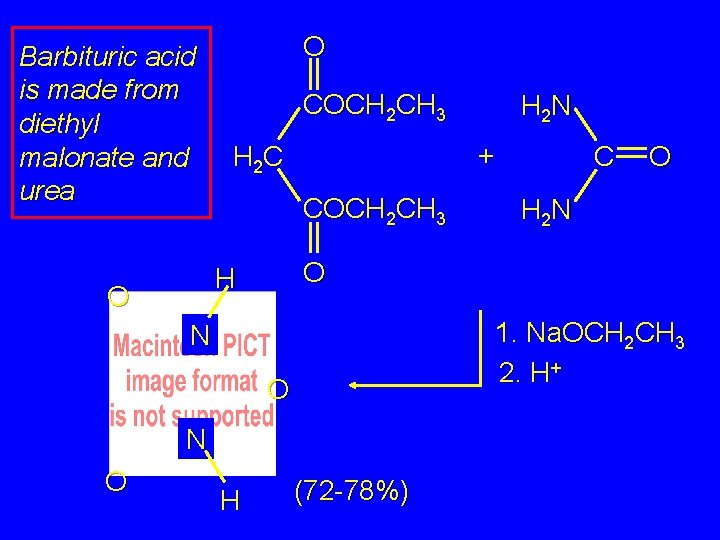
Barbituric acid is made from diethyl malonate and urea O COCH 2 CH 3 + H 2 C COCH 2 CH 3 O N H O H 2 N 1. Na. OCH 2 CH 3 2. H+ N O C O H 2 N (72 -78%)
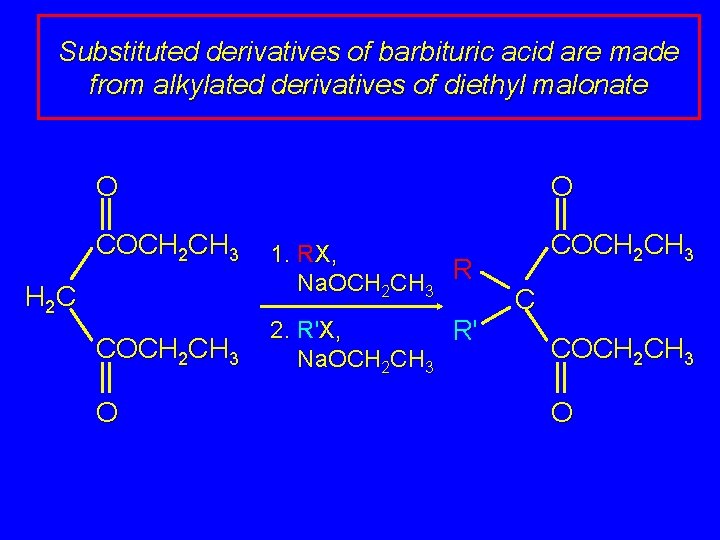
Substituted derivatives of barbituric acid are made from alkylated derivatives of diethyl malonate O COCH 2 CH 3 H 2 C COCH 2 CH 3 O O 1. RX, Na. OCH 2 CH 3 R 2. R'X, R' Na. OCH 2 CH 3 C COCH 2 CH 3 O
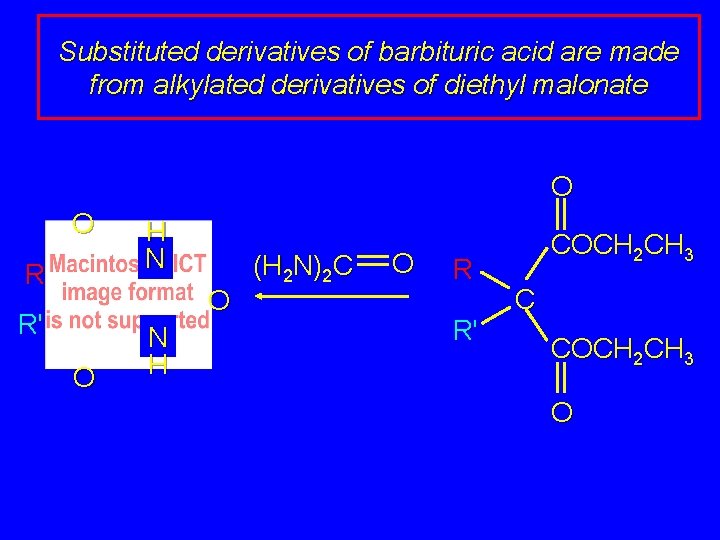
Substituted derivatives of barbituric acid are made from alkylated derivatives of diethyl malonate O O R H N O R' O N H (H 2 N)2 C O R R' COCH 2 CH 3 C COCH 2 CH 3 O
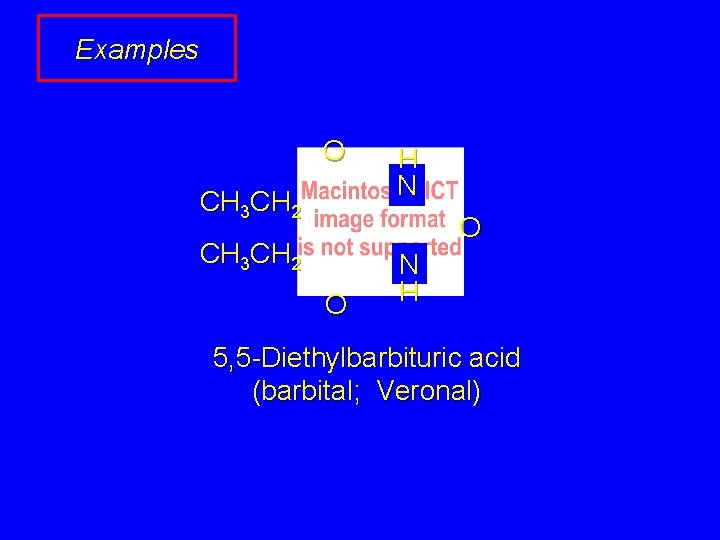
Examples O CH 3 CH 2 H N O CH 3 CH 2 O N H 5, 5 -Diethylbarbituric acid (barbital; Veronal)
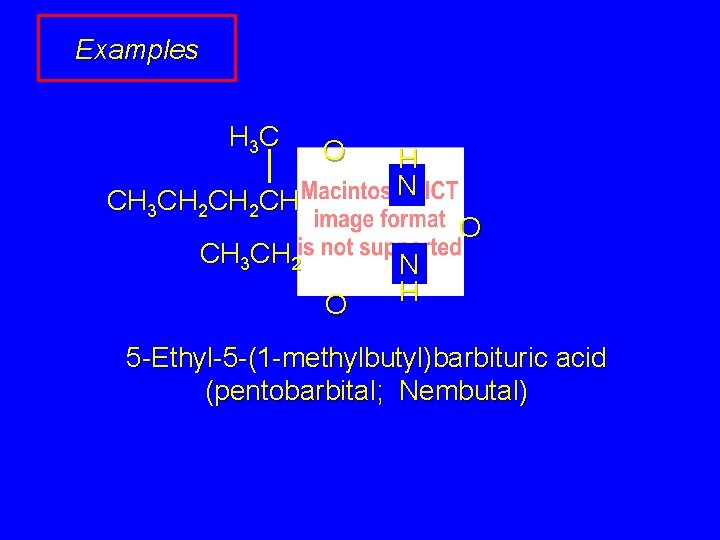
Examples H 3 C O CH 3 CH 2 CH H N O CH 3 CH 2 O N H 5 -Ethyl-5 -(1 -methylbutyl)barbituric acid (pentobarbital; Nembutal)
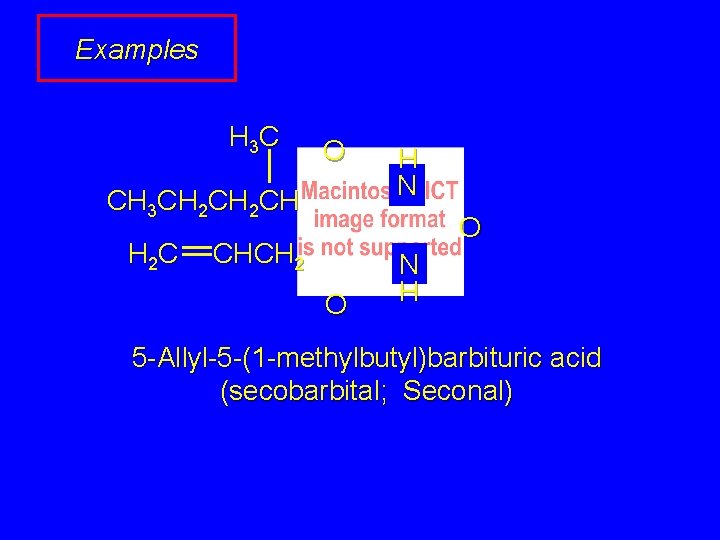
Examples H 3 C O CH 3 CH 2 CH H 2 C H N O CHCH 2 O N H 5 -Allyl-5 -(1 -methylbutyl)barbituric acid (secobarbital; Seconal)

21. 9 Michael Additions of Stabilized Anions
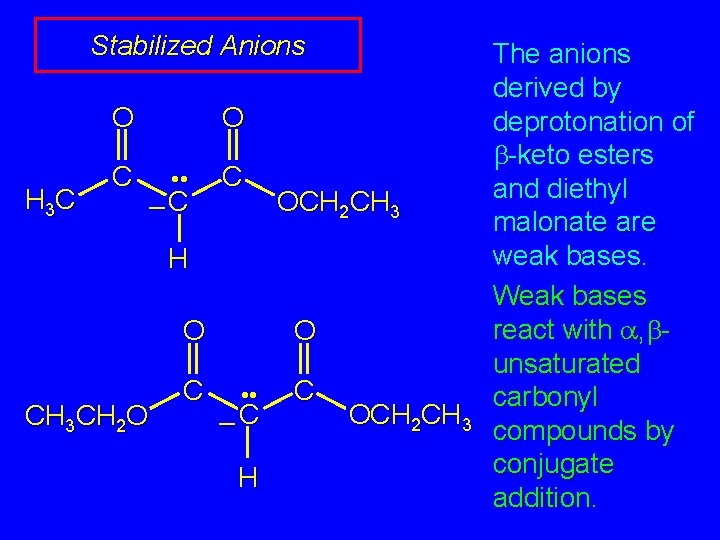
Stabilized Anions O H 3 C C O • • –C C OCH 2 CH 3 H O CH 3 CH 2 O C O • • –C H C OCH 2 CH 3 The anions derived by deprotonation of b-keto esters and diethyl malonate are weak bases. Weak bases react with a, bunsaturated carbonyl compounds by conjugate addition.
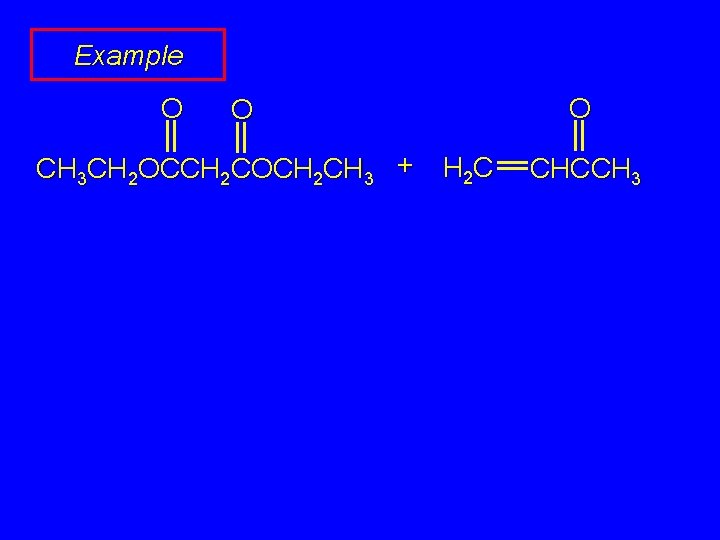
Example O O O CH 3 CH 2 OCCH 2 COCH 2 CH 3 + H 2 C CHCCH 3
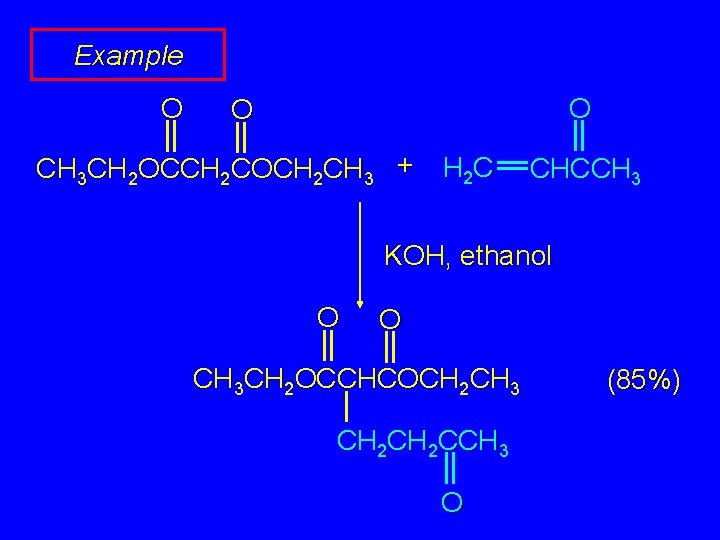
Example O O O CH 3 CH 2 OCCH 2 COCH 2 CH 3 + H 2 C CHCCH 3 KOH, ethanol O O CH 3 CH 2 OCCHCOCH 2 CH 3 CH 2 CCH 3 O (85%)
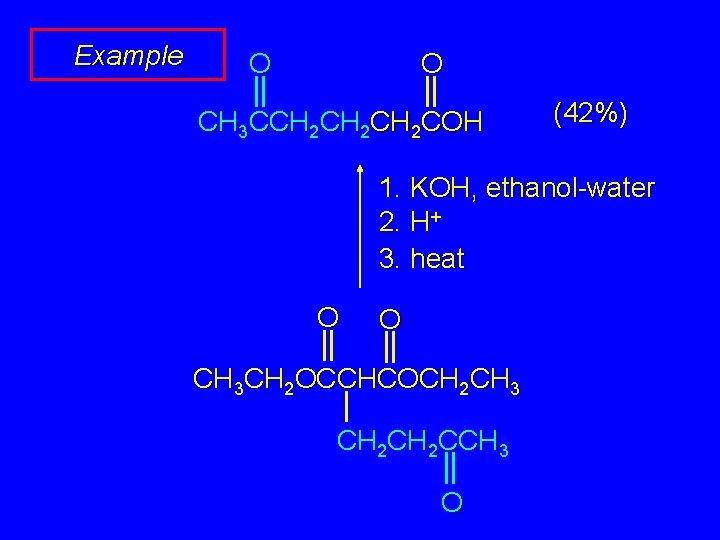
Example O O CH 3 CCH 2 CH 2 COH (42%) 1. KOH, ethanol-water 2. H+ 3. heat O O CH 3 CH 2 OCCHCOCH 2 CH 3 CH 2 CCH 3 O

21. 10 a-Deprotonation of Carbonyl Compounds by Lithium Dialkylamides

Deprotonation of Simple Esters Ethyl acetoacetate (p. Ka ~11) and diethyl malonate (p. Ka ~13) are completely deprotonated by alkoxide bases. Simple esters (such as ethyl acetate) are not completely deprotonated, the enolate reacts with the original ester, and Claisen condensation occurs. Are there bases strong enough to completely deprotonate simple esters, giving ester enolates quantitatively?
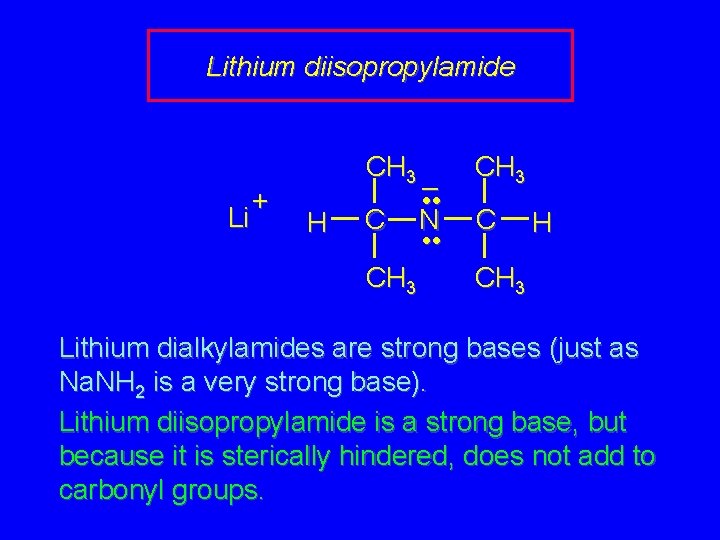
Lithium diisopropylamide Li + CH 3 H C CH 3 – • • N • • CH 3 C H CH 3 Lithium dialkylamides are strong bases (just as Na. NH 2 is a very strong base). Lithium diisopropylamide is a strong base, but because it is sterically hindered, does not add to carbonyl groups.
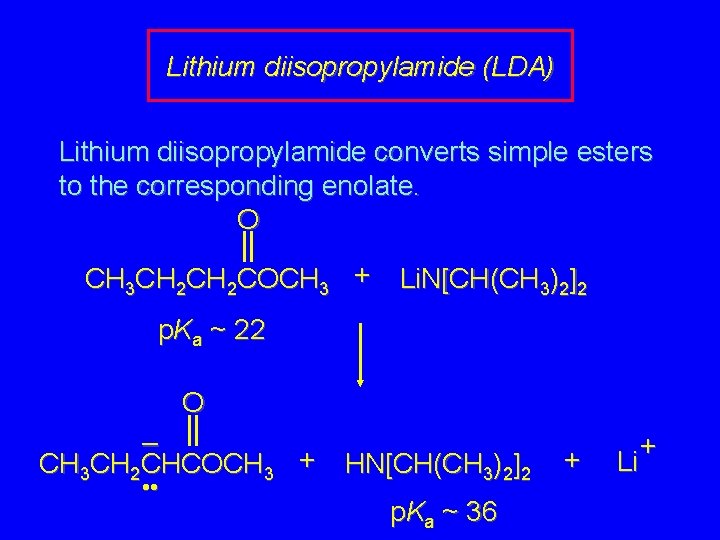
Lithium diisopropylamide (LDA) Lithium diisopropylamide converts simple esters to the corresponding enolate. O CH 3 CH 2 COCH 3 + Li. N[CH(CH 3)2]2 p. Ka ~ 22 O – CH 3 CH 2 CHCOCH 3 + HN[CH(CH 3)2]2 • • p. Ka ~ 36 + Li +
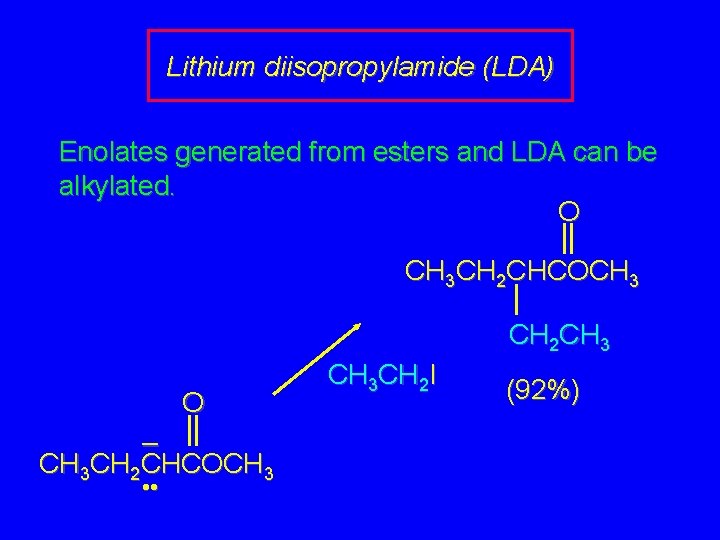
Lithium diisopropylamide (LDA) Enolates generated from esters and LDA can be alkylated. O CH 3 CH 2 CHCOCH 3 CH 2 CH 3 O – CH 3 CH 2 CHCOCH 3 • • CH 3 CH 2 I (92%)
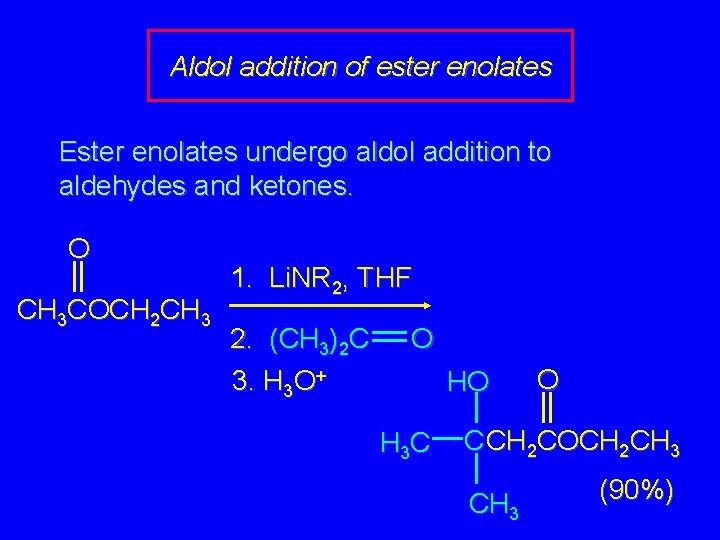
Aldol addition of ester enolates Ester enolates undergo aldol addition to aldehydes and ketones. O CH 3 COCH 2 CH 3 1. Li. NR 2, THF 2. (CH 3)2 C 3. H 3 O+ O HO H 3 C O C CH 2 COCH 2 CH 3 (90%)
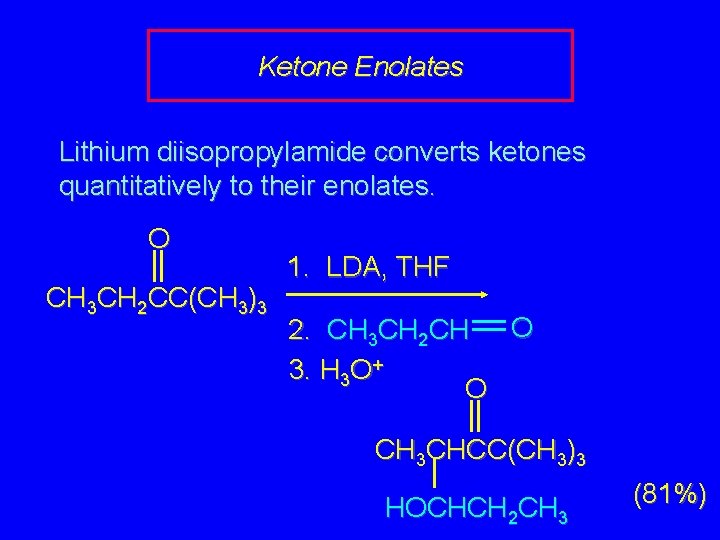
Ketone Enolates Lithium diisopropylamide converts ketones quantitatively to their enolates. O CH 3 CH 2 CC(CH 3)3 1. LDA, THF O 2. CH 3 CH 2 CH 3. H 3 O+ O CH 3 CHCC(CH 3)3 HOCHCH 2 CH 3 (81%)
- Slides: 21