20180814 Adsorption and absorption of hydrogen in Titanium

2018/08/14 Adsorption and absorption of hydrogen in Titanium dioxide Institute of Industrial Science The University of Tokyo K. Fukutani Co-workers: Y. Ohashi, N. Nagatsuka, A. Sakurai S. Ogura, S. Ashihara
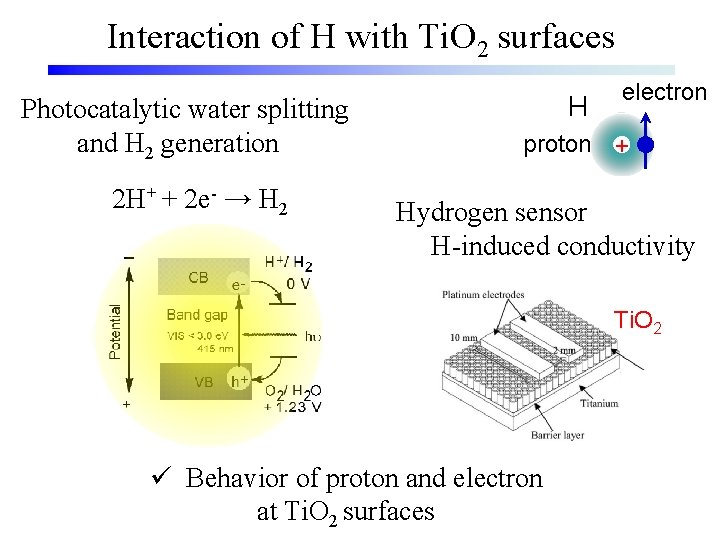
Interaction of H with Ti. O 2 surfaces Photocatalytic water splitting and H 2 generation 2 H+ + 2 e- → H 2 H electron proton + Hydrogen sensor H-induced conductivity Ti. O 2 ü Behavior of proton and electron at Ti. O 2 surfaces
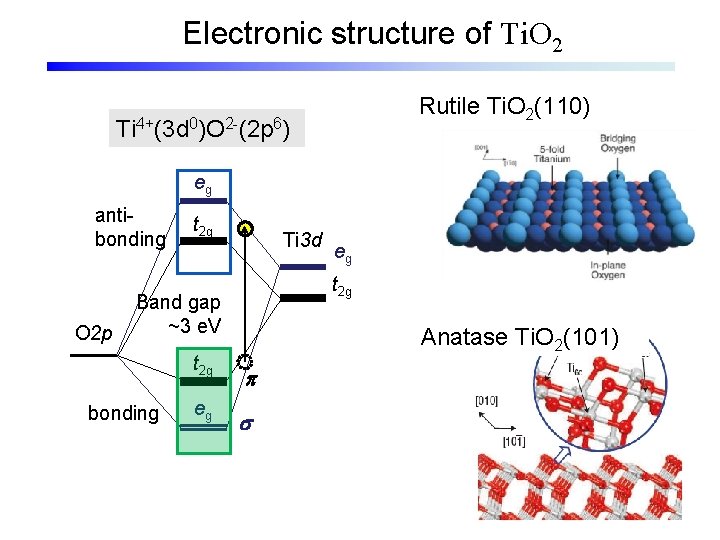
Electronic structure of Ti. O 2 Rutile Ti. O 2(110) Ti 4+(3 d 0)O 2 -(2 p 6) eg antibonding O 2 p t 2 g Ti 3 d t 2 g Band gap ~3 e. V t 2 g bonding eg eg Anatase Ti. O 2(101) p s
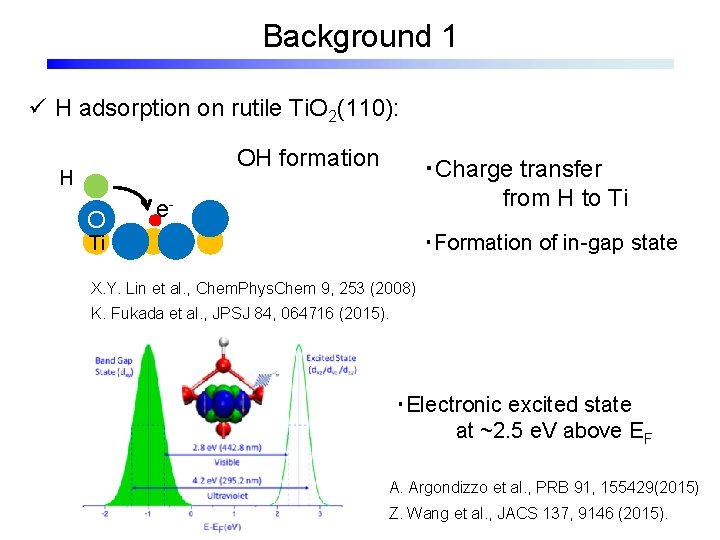
Background 1 ü H adsorption on rutile Ti. O 2(110): OH formation H O ・Charge transfer from H to Ti e- ・Formation of in-gap state Ti X. Y. Lin et al. , Chem. Phys. Chem 9, 253 (2008) K. Fukada et al. , JPSJ 84, 064716 (2015). ・Electronic excited state at ~2. 5 e. V above EF A. Argondizzo et al. , PRB 91, 155429(2015) Z. Wang et al. , JACS 137, 9146 (2015).

Background 2 ü Hydrogenation of Ti. O 2 Enhancement of solar absorption • High pressure • Plasma • Ion implantation Importance of disorder Wang et al. , Adv. Func. Mater. 23, 5444 (2013). H O Ti H implantation at 40 ke. V Nandasiri et al. , JPCL 6, 4627 (2015)
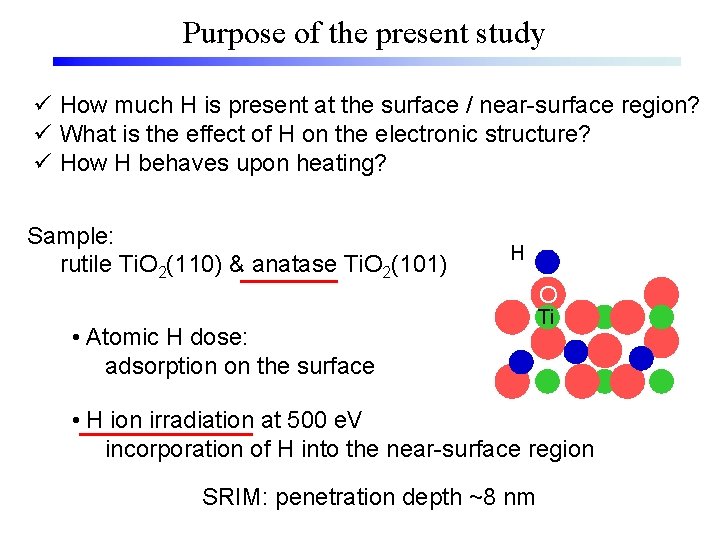
Purpose of the present study ü How much H is present at the surface / near-surface region? ü What is the effect of H on the electronic structure? ü How H behaves upon heating? Sample: rutile Ti. O 2(110) & anatase Ti. O 2(101) H • Atomic H dose: adsorption on the surface O Ti • H ion irradiation at 500 e. V incorporation of H into the near-surface region SRIM: penetration depth ~8 nm
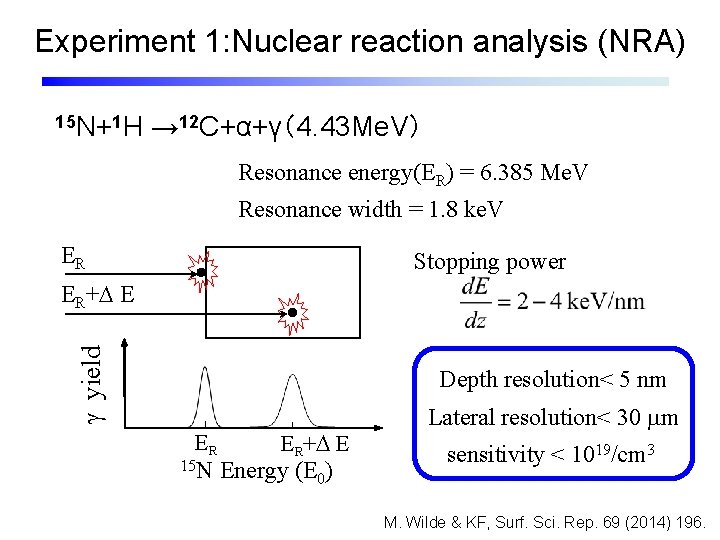
Experiment 1: Nuclear reaction analysis (NRA) 15 N+1 H → 12 C+α+γ(4. 43 Me. V) Resonance energy(ER) = 6. 385 Me. V Resonance width = 1. 8 ke. V ER Stopping power g yield ER+D E Depth resolution< 5 nm ER ER+D E 15 N Energy (E ) 0 Lateral resolution< 30 mm sensitivity < 1019/cm 3 M. Wilde & KF, Surf. Sci. Rep. 69 (2014) 196.

Experiment 2: photoemission Ti: Saphire He I (21. 22 e. V) Laser (~ 100 fs) 3. 4 -4. 0 e. V l/2 plate e analyzer BBO (4 HG) 1. 55 e. V BBO (OPA) 0. 851. 0 e. V 0. 550. 70 e. V BBO (SHG) 1. 7 -2. 0 e. V ・He. I: 1 -photon PES occupied state ・Laser: 2 -photon PES unoccupied state

Electronic structure of Ti. O 2 Rutile Ti. O 2(110) Ti 4+(3 d 0)O 2 -(2 p 6) eg antibonding O 2 p t 2 g Ti 3 d t 2 g Band gap ~3 e. V t 2 g bonding eg eg p s Anatase Ti. O 2(101)
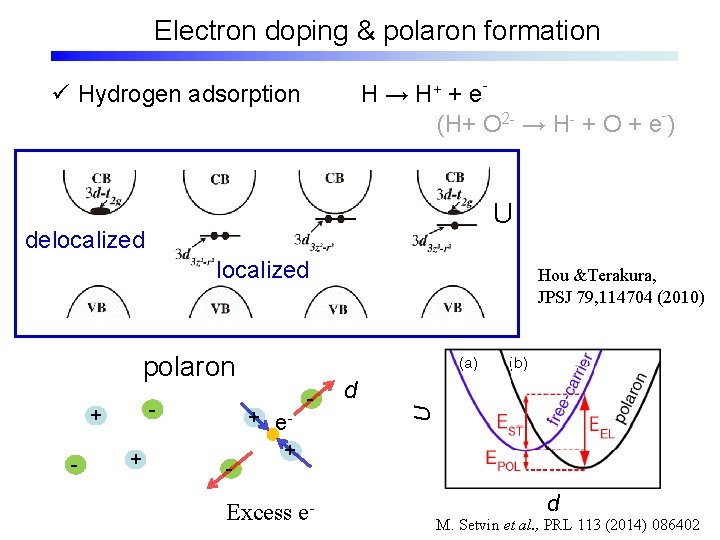
Electron doping & polaron formation H → H+ + e(H+ O 2 - → H- + O + e-) ü Hydrogen adsorption U delocalized polaron - + - + e+ - Excess e- Hou &Terakura, JPSJ 79, 114704 (2010) d M. Setvin et al. , PRL 113 (2014) 086402
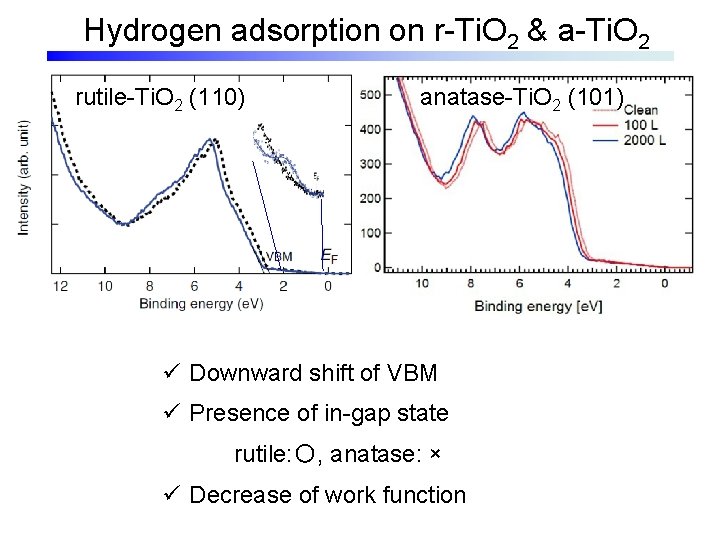
Hydrogen adsorption on r-Ti. O 2 & a-Ti. O 2 rutile-Ti. O 2 (110) anatase-Ti. O 2 (101) ü Downward shift of VBM ü Presence of in-gap state rutile: 〇, anatase: × ü Decrease of work function
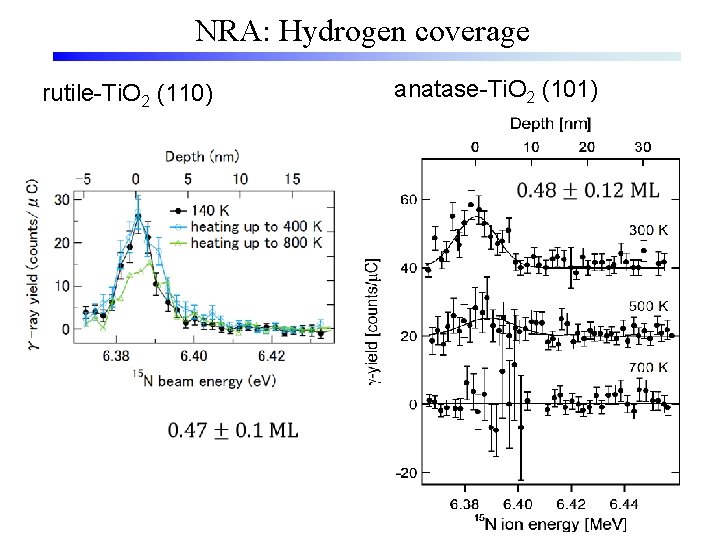
NRA: Hydrogen coverage rutile-Ti. O 2 (110) anatase-Ti. O 2 (101)
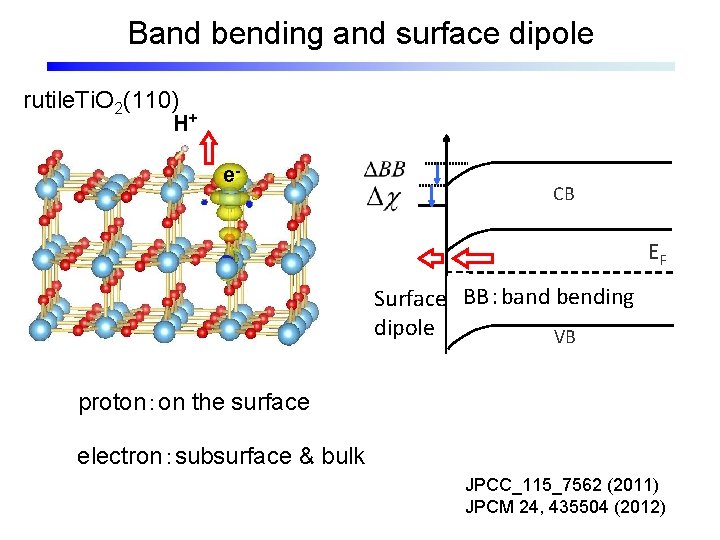
Band bending and surface dipole rutile. Ti. O 2(110) H+ e- CB EF Surface BB:band bending dipole VB proton:on the surface electron:subsurface & bulk JPCC_115_7562 (2011) JPCM 24, 435504 (2012)
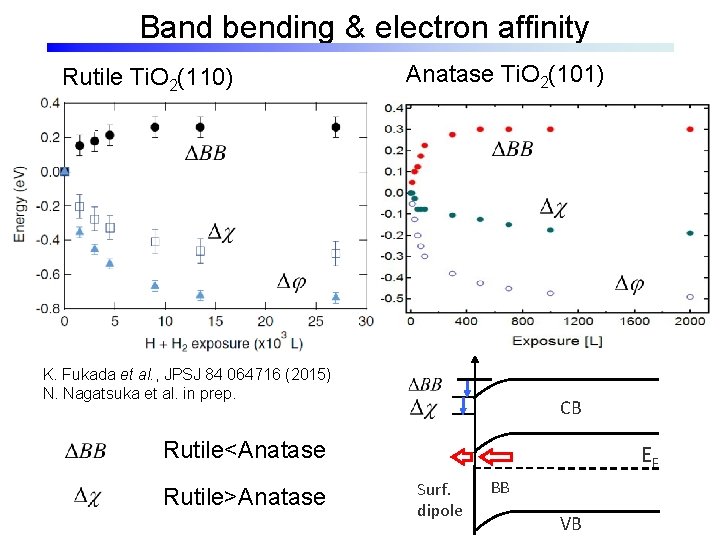
Band bending & electron affinity Rutile Ti. O 2(110) Anatase Ti. O 2(101) K. Fukada et al. , JPSJ 84 064716 (2015) N. Nagatsuka et al. in prep. CB Rutile<Anatase Rutile>Anatase EF Surf. dipole BB VB
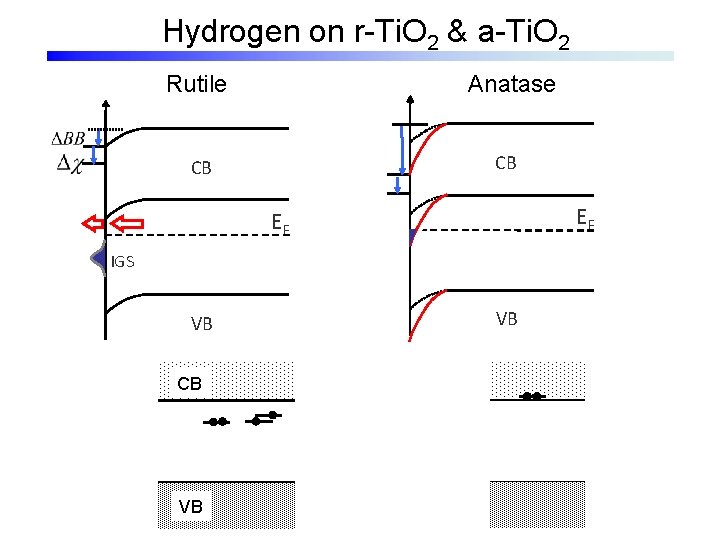
Hydrogen on r-Ti. O 2 & a-Ti. O 2 Anatase Rutile CB CB EF EF IGS VB CB VB VB
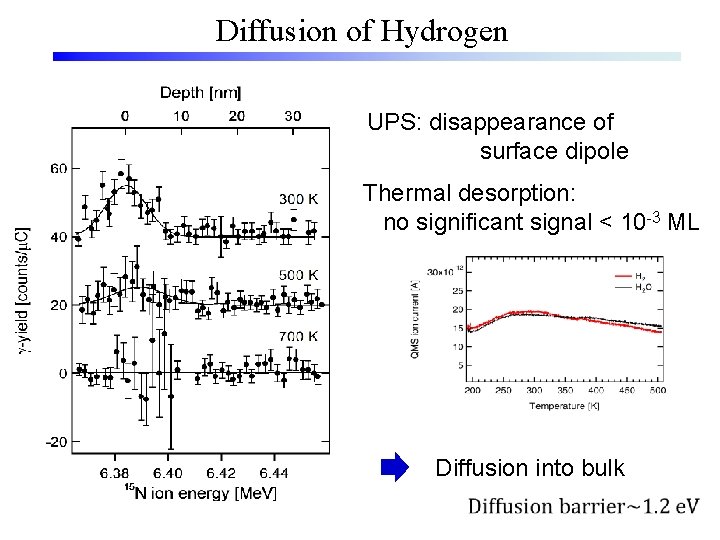
Diffusion of Hydrogen UPS: disappearance of surface dipole Thermal desorption: no significant signal < 10 -3 ML Diffusion into bulk
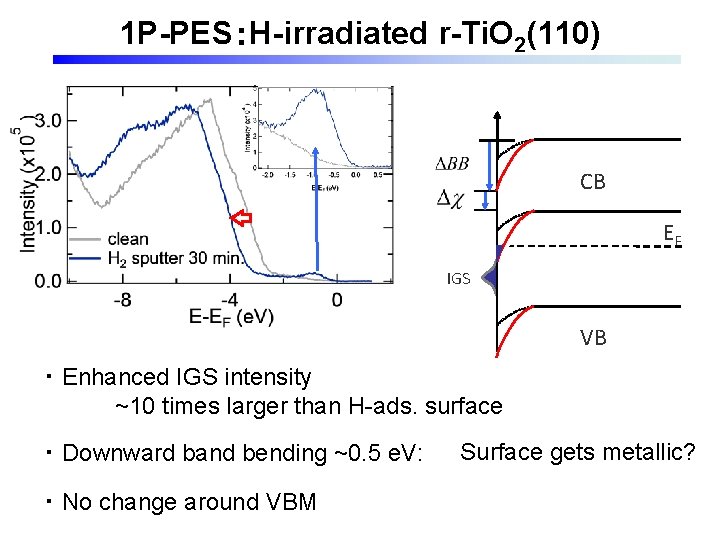
1 P-PES:H-irradiated r-Ti. O 2(110) CB EF IGS VB ・ Enhanced IGS intensity ~10 times larger than H-ads. surface ・ Downward band bending ~0. 5 e. V: ・ No change around VBM Surface gets metallic?
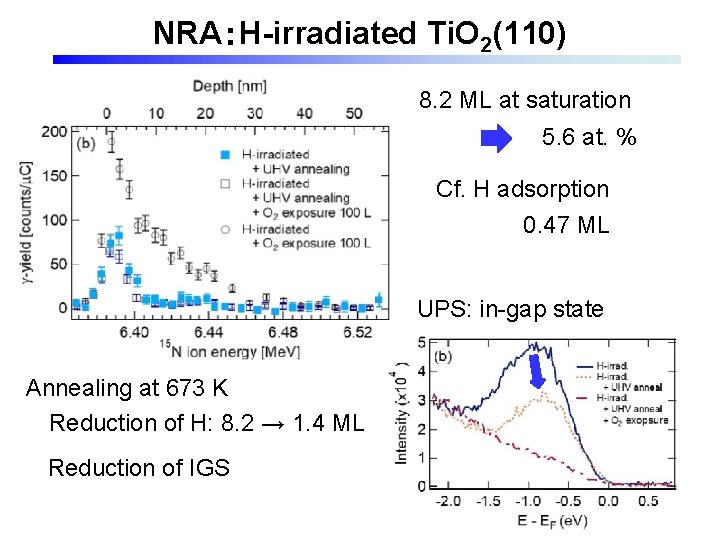
NRA:H-irradiated Ti. O 2(110) 8. 2 ML at saturation 5. 6 at. % Cf. H adsorption 0. 47 ML UPS: in-gap state Annealing at 673 K Reduction of H: 8. 2 → 1. 4 ML Reduction of IGS
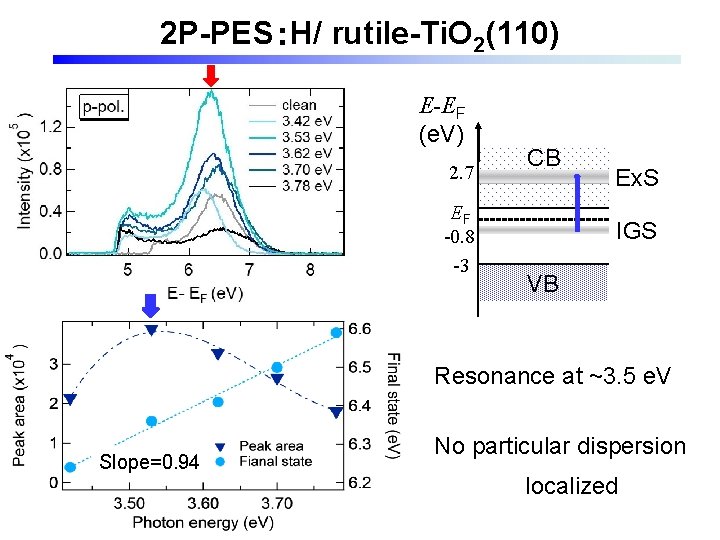
2 P-PES:H/ rutile-Ti. O 2(110) E-EF (e. V) 2. 7 EF -0. 8 -3 CB Ex. S IGS VB Resonance at ~3. 5 e. V 傾き=0. 94 Slope=0. 94 No particular dispersion localized

Intensity (arb. u. ) 2 P-PES:H-irradiated Ti. O 2(110) Resonance at ~3. 5 & ~3. 7 e. V Peak position (e. V) Photon energy (e. V) Slope~1 Photon energy (e. V)
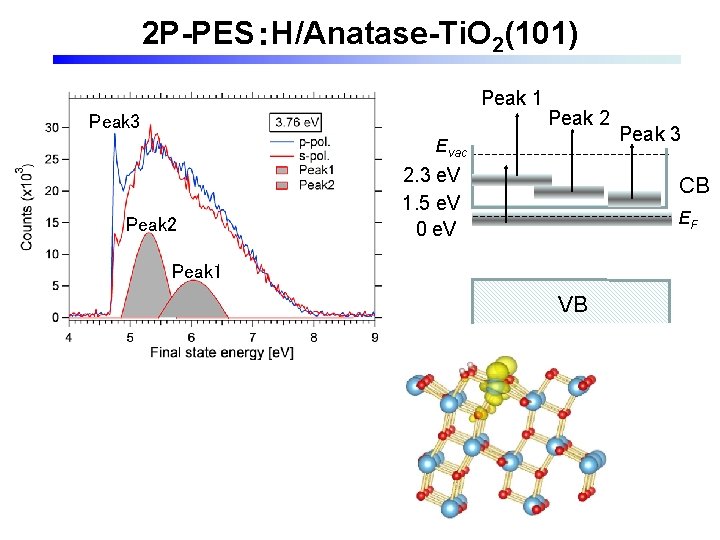
2 P-PES:H/Anatase-Ti. O 2(101) Peak 1 Peak 3 Peak 2 Evac Peak 2 2. 3 e. V 1. 5 e. V 0 e. V Peak 3 CB EF Peak 1 VB
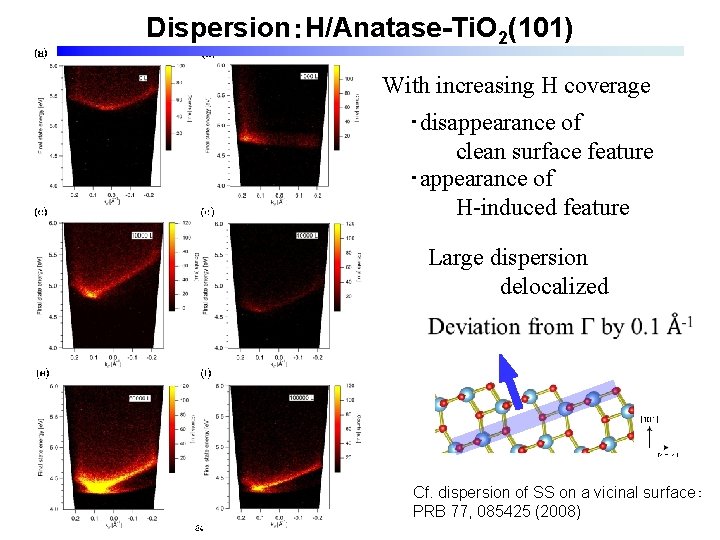
Dispersion:H/Anatase-Ti. O 2(101) With increasing H coverage ・disappearance of clean surface feature ・appearance of H-induced feature Large dispersion delocalized Cf. dispersion of SS on a vicinal surface: PRB 77, 085425 (2008)
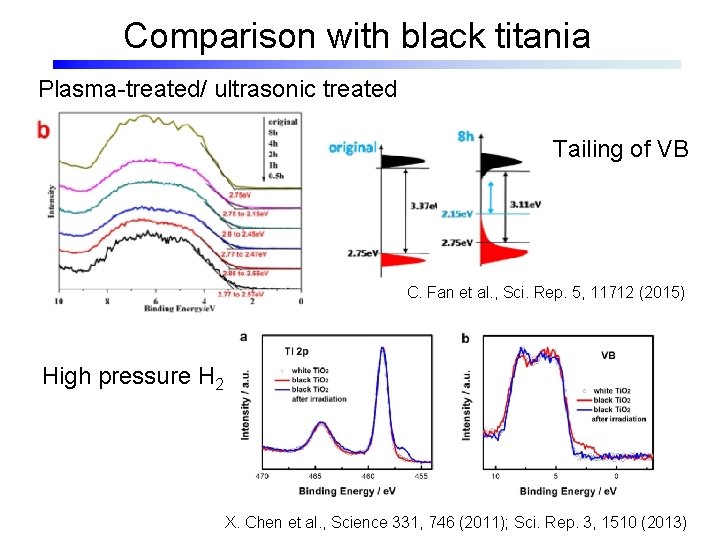
Comparison with black titania Plasma-treated/ ultrasonic treated Tailing of VB C. Fan et al. , Sci. Rep. 5, 11712 (2015) High pressure H 2 X. Chen et al. , Science 331, 746 (2011); Sci. Rep. 3, 1510 (2013)
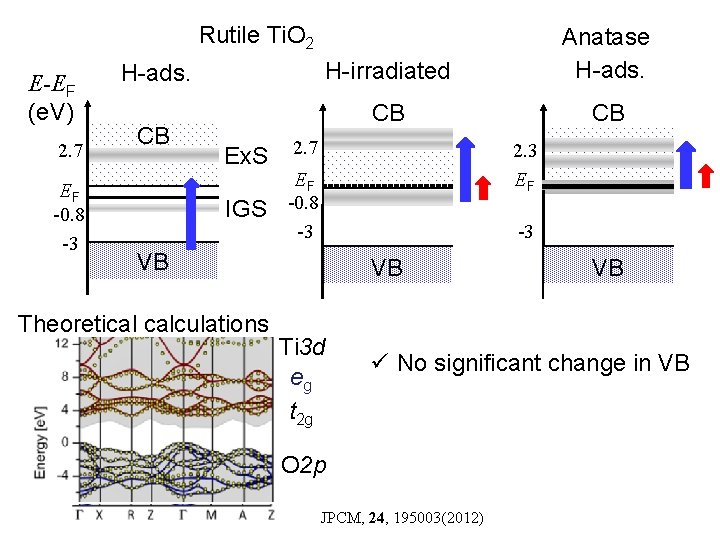
Rutile Ti. O 2 E-EF (e. V) 2. 7 EF -0. 8 -3 H-ads. CB Ex. S IGS H-irradiated Anatase H-ads. CB CB 2. 7 2. 3 EF EF -0. 8 -3 -3 VB Theoretical calculations VB Ti 3 d eg t 2 g VB ü No significant change in VB O 2 p JPCM, 24, 195003(2012)
Summary Hydrogen adsorption and absorption at rutile Ti. O 2(110) and anatase Ti. O 2(101) surfaces with NRA, 1 P-PES and 2 P-PES 1. H adsorption: saturation coverage=0. 48 ML el. ground state rutile: in-gap state, anatase: no in-gap state (metallic) el. excited state above EF rutile: ~2. 7 e. V (localized), anatase: ~2. 3 e. V (extended) 2. H irradiation at 500 e. V concentration: ~5 at. % within ~10 nm enhancement of In-gap state (~10 times larger) thermal diffusion of H
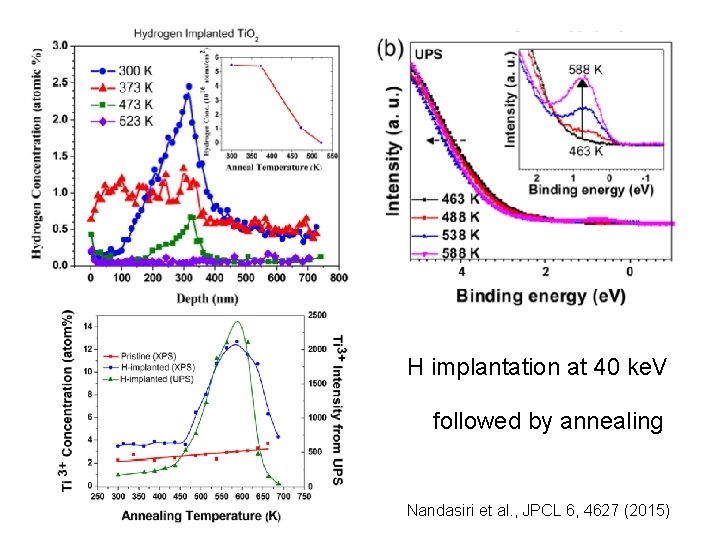
H implantation at 40 ke. V followed by annealing Nandasiri et al. , JPCL 6, 4627 (2015)
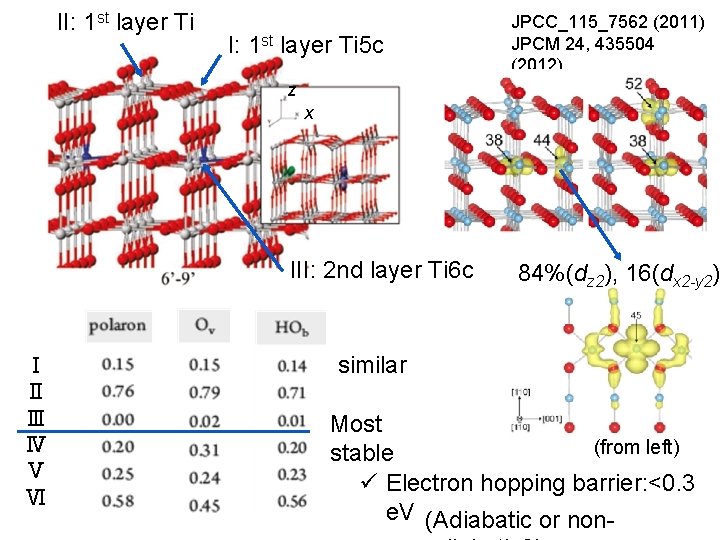
II: 1 st layer Ti 5 c JPCC_115_7562 (2011) JPCM 24, 435504 (2012) z x III: 2 nd layer Ti 6 c Ⅰ Ⅱ Ⅲ Ⅳ Ⅴ Ⅵ 84%(dz 2), 16(dx 2 -y 2) similar Most (from left) stable ü Electron hopping barrier: <0. 3 e. V (Adiabatic or non-
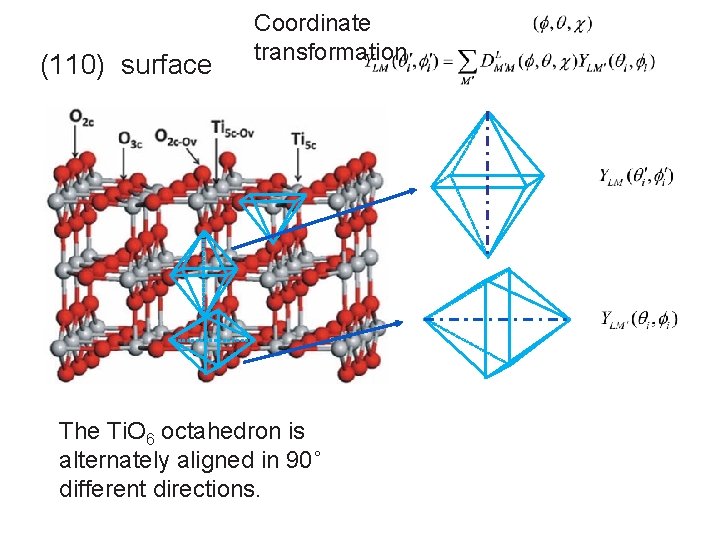
(110) surface Coordinate transformation The Ti. O 6 octahedron is alternately aligned in 90˚ different directions.
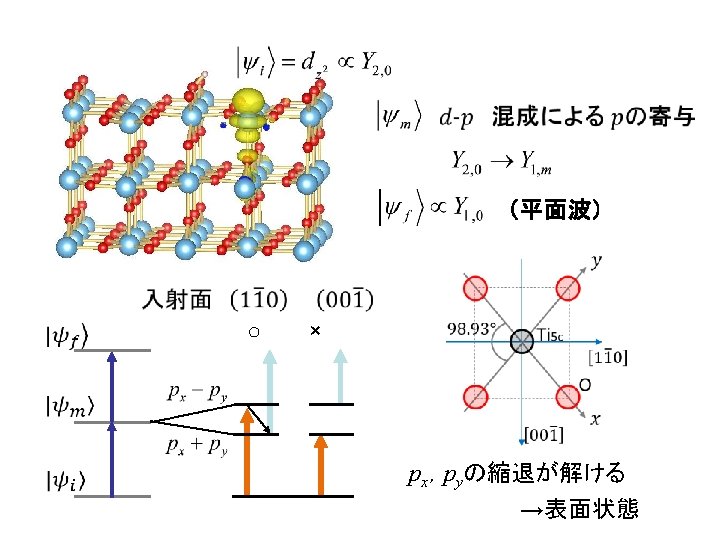
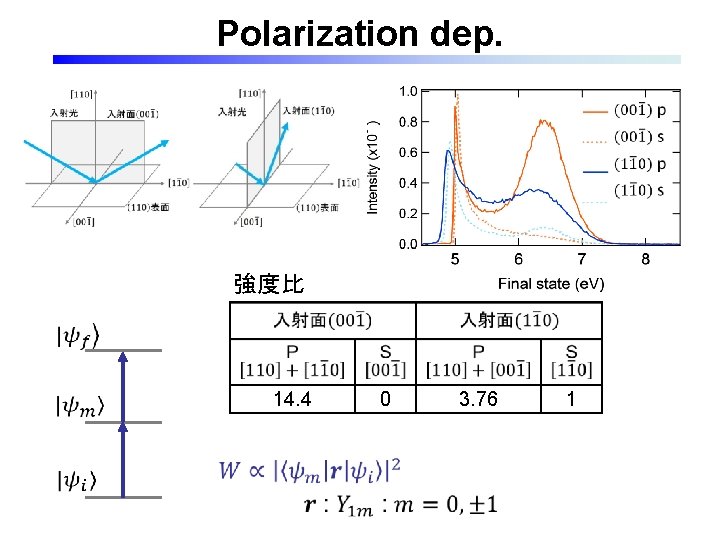
Polarization dep. 強度比 14. 4 0 3. 76 1
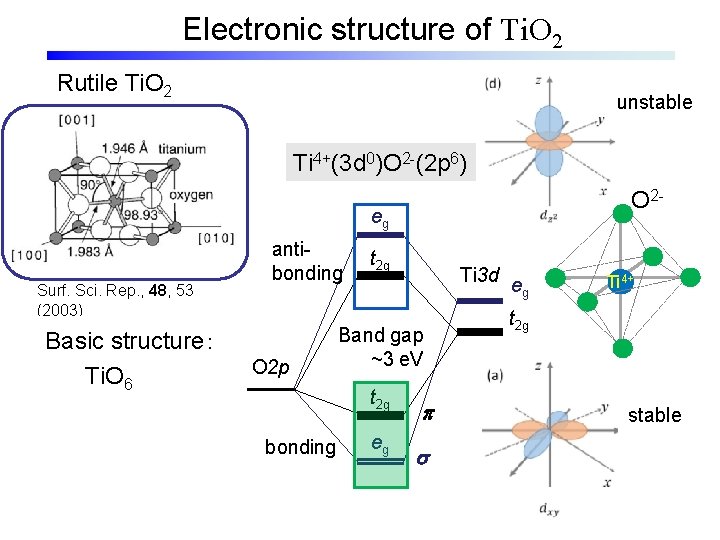
Electronic structure of Ti. O 2 Rutile Ti. O 2 unstable Ti 4+(3 d 0)O 2 -(2 p 6) O 2 - eg Surf. Sci. Rep. , 48, 53 (2003) Basic structure: Ti. O 6 antibonding O 2 p t 2 g Ti 3 d Band gap ~3 e. V t 2 g bonding eg p s eg Ti 4+ t 2 g stable
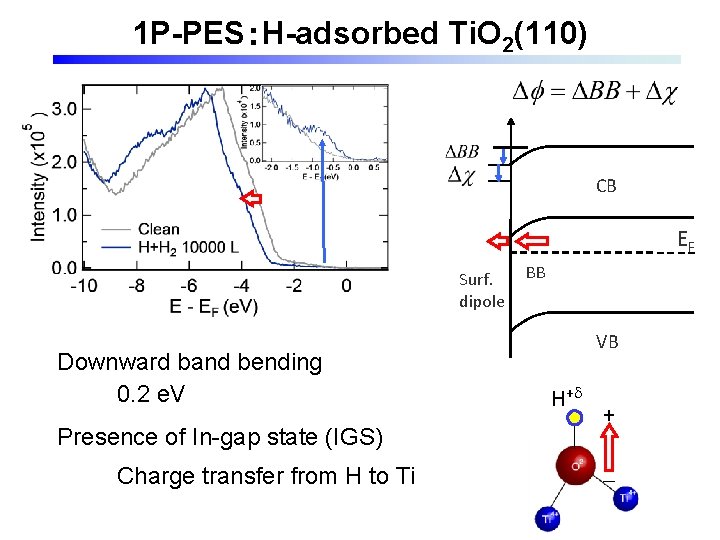
1 P-PES:H-adsorbed Ti. O 2(110) CB EF Surf. dipole Downward band bending 0. 2 e. V Presence of In-gap state (IGS) Charge transfer from H to Ti BB VB H+d + -
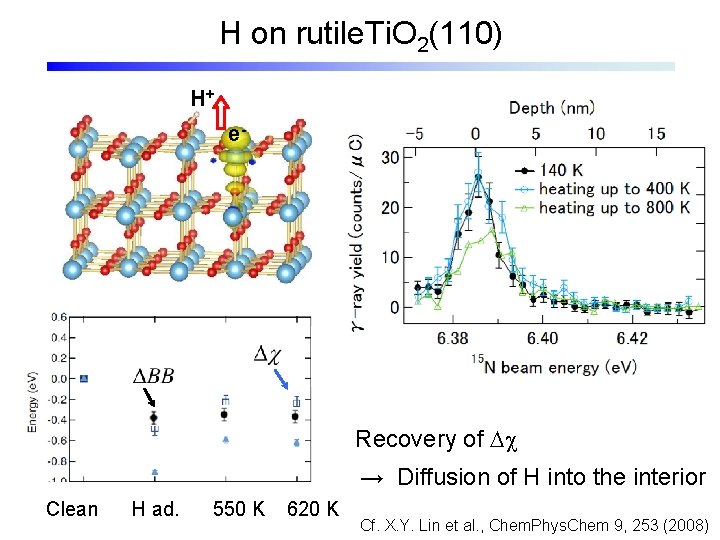
H on rutile. Ti. O 2(110) H+ e- Recovery of Dc → Diffusion of H into the interior Clean H ad. 550 K 620 K Cf. X. Y. Lin et al. , Chem. Phys. Chem 9, 253 (2008)
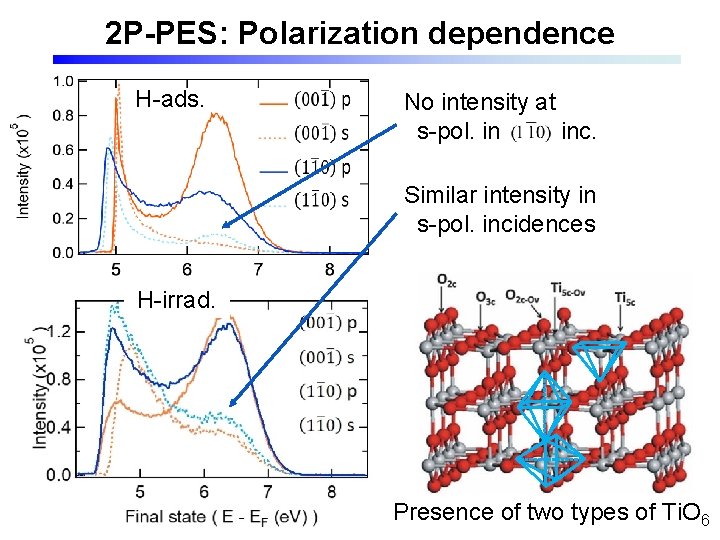
2 P-PES: Polarization dependence H-ads. No intensity at s-pol. in inc. Similar intensity in s-pol. incidences H-irrad. Presence of two types of Ti. O 6
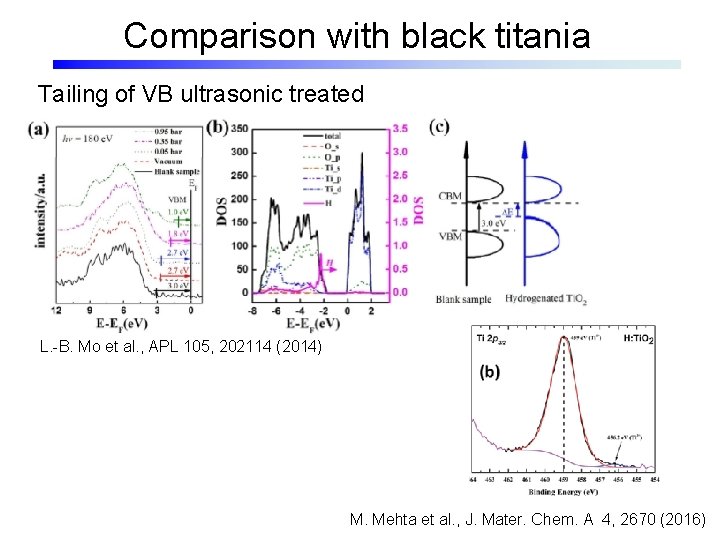
Comparison with black titania Tailing of VB ultrasonic treated L. -B. Mo et al. , APL 105, 202114 (2014) M. Mehta et al. , J. Mater. Chem. A 4, 2670 (2016)
- Slides: 35