2015 NHSN Training Teresa Fox CIC Quality Improvement






















- Slides: 70

2015 NHSN Training Teresa Fox, CIC Quality Improvement Advisor teresa. fox@area-g. hcqis. org

Objectives Discuss New Definitions and Modifications for 2015 Review of Reporting for Lab. ID Events Review of Reporting for CAUTI 2

HAI Surveillance in the Current U. S. Environment Public Reporting Pay for Performance 3 Implications Changes in NHSN’s purposes, infrastructure and operations New scrutiny of HAI case criteria Increasing attention to data quality Pressure to HAI definitions and move to electronic HAI detection and reporting

Implications of Public Reporting and Pay for Performance New scrutiny of HAI definitions and case studies • Updates of definitions and case criteria that reflect user’s concerns about misclassification of some events Heightened emphasis on data validation • Assistance to states and CMS for validation Pressure to simplify HAI definitions and data requirements and move to electronic HAI detection and reporting • Revise definitions and data requirements in ways to reduce complexity, maintain clinical relevance, and avoid potential case misclassification • Accelerate use of electronic healthcare data for event detection and reporting purposes 4

NHSN Plans for 2015 and Beyond New baseline year for ALL HAIs • Implementation of new definitions and criteria changes for several HAIs concurrently and retaining those previously established • Allow data from single calendar year as the new baseline for SIRs • Provide baseline data for calculating SIRs for 2016 and the following years • Potential to recalculate 2015 SIRs from the 2015 baseline population Future changes (3 -5 years) will be driven by increasing healthcare data availability via electronic methods and increasing EHRs 5

2015 CMS Reporting Requirements-Acute Hospitals CLABSI • Adult, pediatric and neonatal ICUs • Adult and pediatric medical wards, surgical wards & med-surgical wards CAUTI • Adult and pediatric ICUs • Adult and pediatric medical wards, surgical wards & med-surgical wards SSI • Inpatient colon and abdominal hysterectomy procedures 6

2015 CMS Reporting Requirements-Acute Hospitals (cont’d) MRSA Bacteremia and CDI Lab. ID Events • Facility-wide inpatients HCP Influenza Vaccination Summary • Inpatient and outpatient healthcare personnel Medicare Beneficiary Number • All events (numerators) from Medicare patients 7

Major Definition Changes for Specific Types of Infections BSI, Pneu, SSI, UTI, VAE • Removed from Chapter 17 • Found in separate, dedicated chapters • Chapters cover both deviceassociated and non-device associated BRON • Removed entirely from NHSN surveillance 8 UTI • Major changes to definitions-covered in UTI presentation Secondary BSI Attribution • Major changes to definition for Secondary Bloodstream Infections • Reinforced in CLABSI presentation Clostridium difficile infection

New Definitions and Modifications Infection Window Period *+ Date of Event * • Present on Admission Infections (POAs) *+ • If housed in ED prior to admission- date of Admission is date when moved to inpatient location • Healthcare Associated Infections (HAIs) *+ Repeat Infection Timeframe (RIT) *+ Secondary BSI Attribution Period *+ Pathogen assignment (part of RIT) *+ * Does not apply to VAE, or Lab. ID Events # Does not apply to SSI 9

2015 Removed Criteria Gap Days to determine criterion met Logical pathogens to determine secondary BSI Date of Event- when last element of criteria is met 10 DELETE

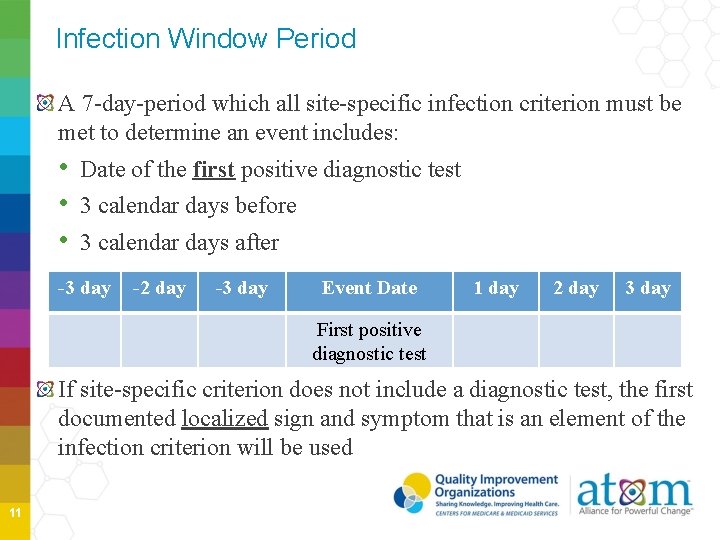
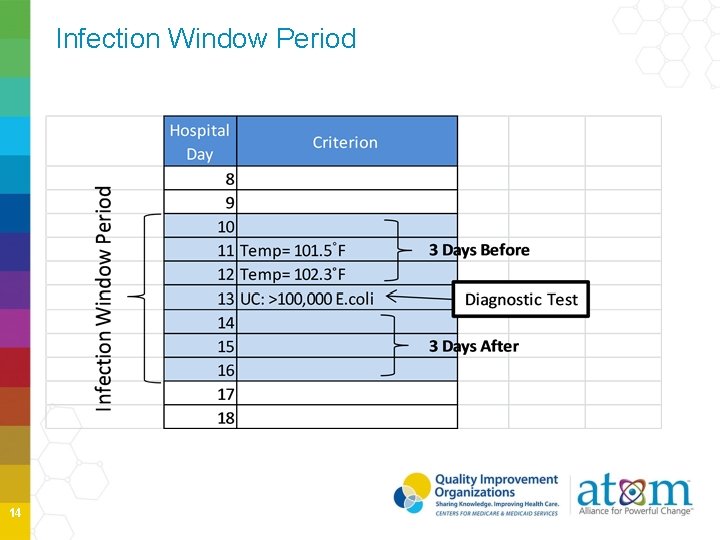
Infection Window Period A 7 -day-period which all site-specific infection criterion must be met to determine an event includes: • Date of the first positive diagnostic test • 3 calendar days before • 3 calendar days after -3 day -2 day -3 day Event Date 1 day 2 day 3 day First positive diagnostic test If site-specific criterion does not include a diagnostic test, the first documented localized sign and symptom that is an element of the infection criterion will be used 11

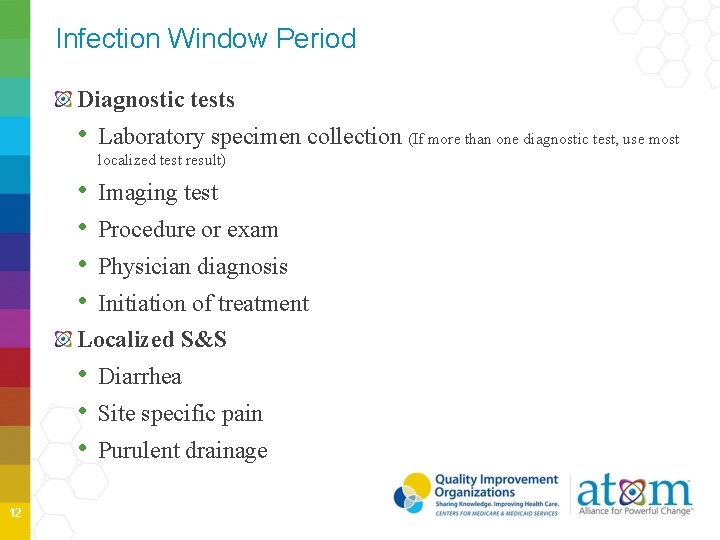
Infection Window Period Diagnostic tests • Laboratory specimen collection (If more than one diagnostic test, use most localized test result) • • Imaging test Procedure or exam Physician diagnosis Initiation of treatment Localized S&S • Diarrhea • Site specific pain • Purulent drainage 12

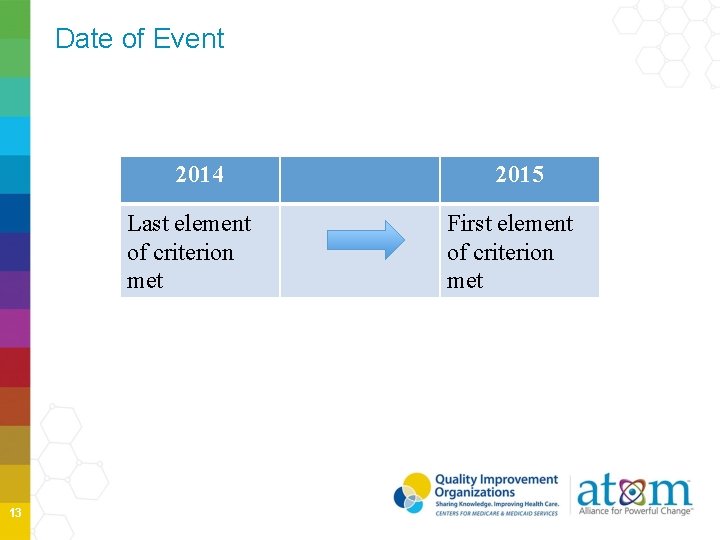
Date of Event 2014 Last element of criterion met 13 2015 First element of criterion met

Infection Window Period 14

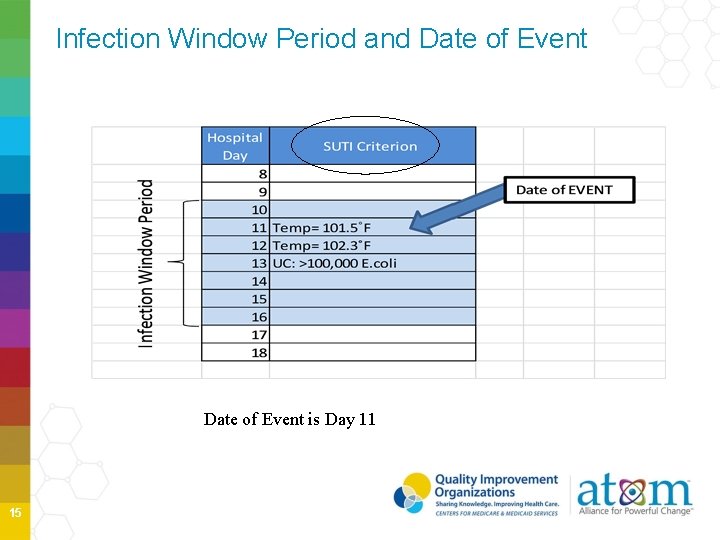
Infection Window Period and Date of Event is Day 11 15

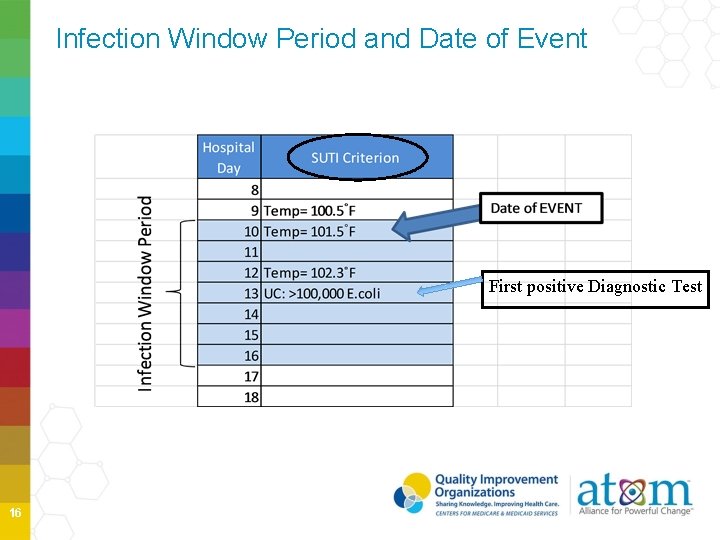
Infection Window Period and Date of Event First positive Diagnostic Test 16

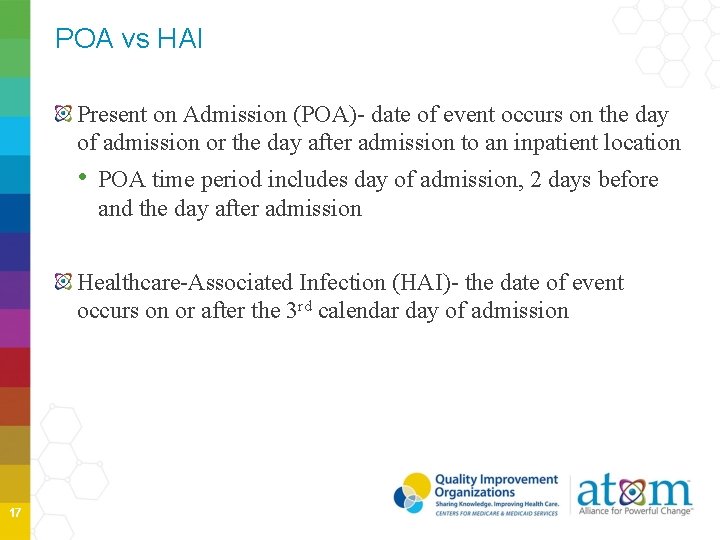
POA vs HAI Present on Admission (POA)- date of event occurs on the day of admission or the day after admission to an inpatient location • POA time period includes day of admission, 2 days before and the day after admission Healthcare-Associated Infection (HAI)- the date of event occurs on or after the 3 rd calendar day of admission 17

Determining New vs. Continuation of Infections 18 2014 2015 Continuation of symptoms or treatment at time of next infection v Subjective v. Undocumented treatment target Repeat Infection Timeframe (RIT) v. Objective v. No interpretation of treatment purposes v. Reduces IP labor resources

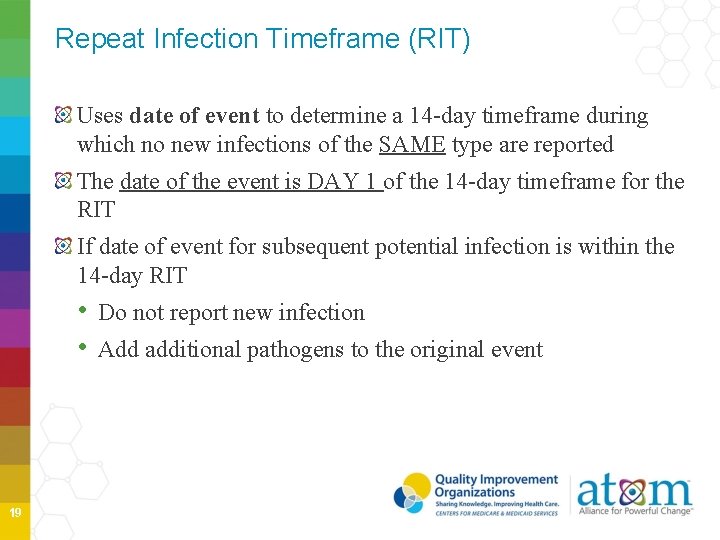
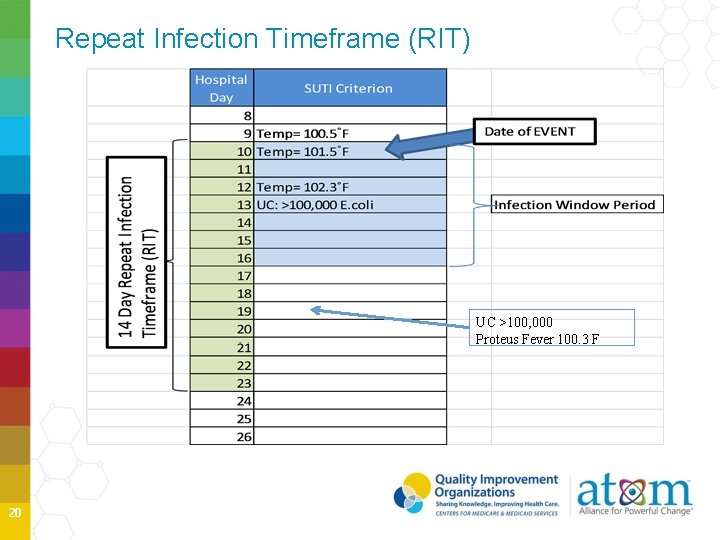
Repeat Infection Timeframe (RIT) Uses date of event to determine a 14 -day timeframe during which no new infections of the SAME type are reported The date of the event is DAY 1 of the 14 -day timeframe for the RIT If date of event for subsequent potential infection is within the 14 -day RIT • Do not report new infection • Add additional pathogens to the original event 19

Repeat Infection Timeframe (RIT) UC >100, 000 Proteus Fever 100. 3 F 20

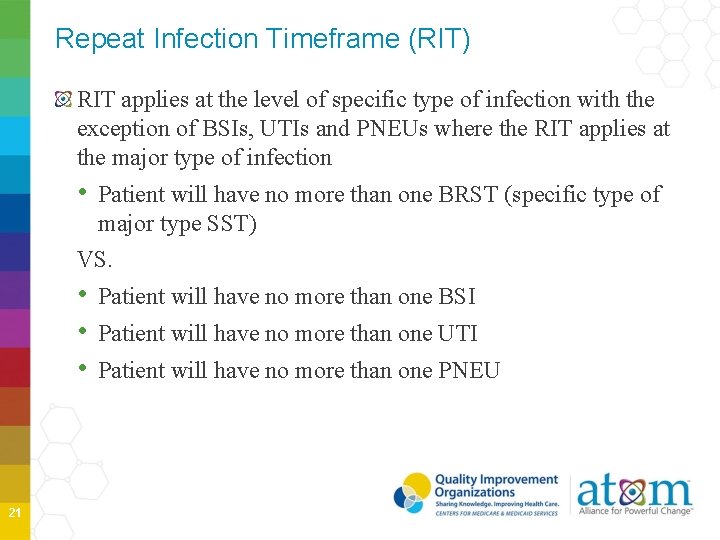
Repeat Infection Timeframe (RIT) RIT applies at the level of specific type of infection with the exception of BSIs, UTIs and PNEUs where the RIT applies at the major type of infection • Patient will have no more than one BRST (specific type of major type SST) VS. • Patient will have no more than one BSI • Patient will have no more than one UTI • Patient will have no more than one PNEU 21

Pathogen Assignment Additional eligible pathogens identified within the RIT are added to the event Pathogen exclusions for specific infection definitions also apply to secondary BSI pathogen assignment • UTI- yeast • Pneu- yeast, coag. negative Staph, Enterococcus unless isolated from lung tissue or pleural fluid; yeast is included for PNU 3 (immunocomprised patients) • Fungal pathogens Excluded pathogens must be attributed to another site infection to be reported as a secondary BSI or it is identified as a primary BSI 22

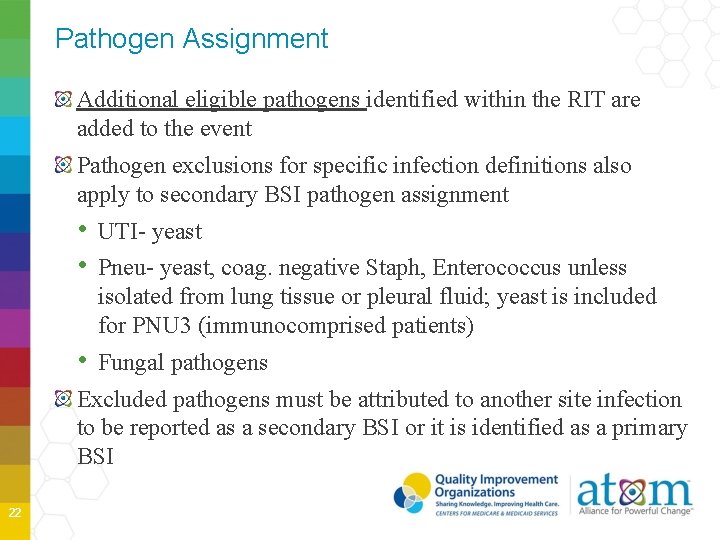
RIT MAJOR TYPE SPECIFIC TYPE Skin and Soft Tissue Infection BRST May have more than one in RIT Burn Circ Decu Skin UTI SUTI ABUTI 23 OR

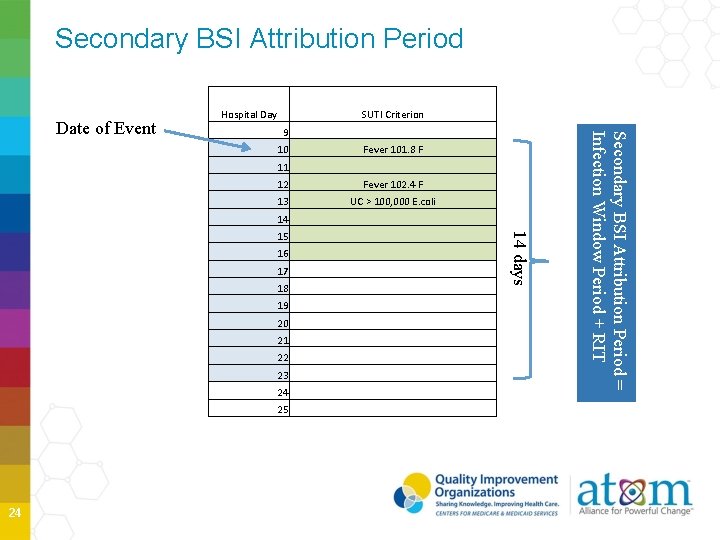
Secondary BSI Attribution Period 9 10 Fever 101. 8 F 11 12 Fever 102. 4 F 13 UC > 100, 000 E. coli 14 15 16 17 18 19 20 21 22 23 24 25 14 days 24 SUTI Criterion Secondary BSI Attribution Period = Infection Window Period + RIT Date of Event Hospital Day

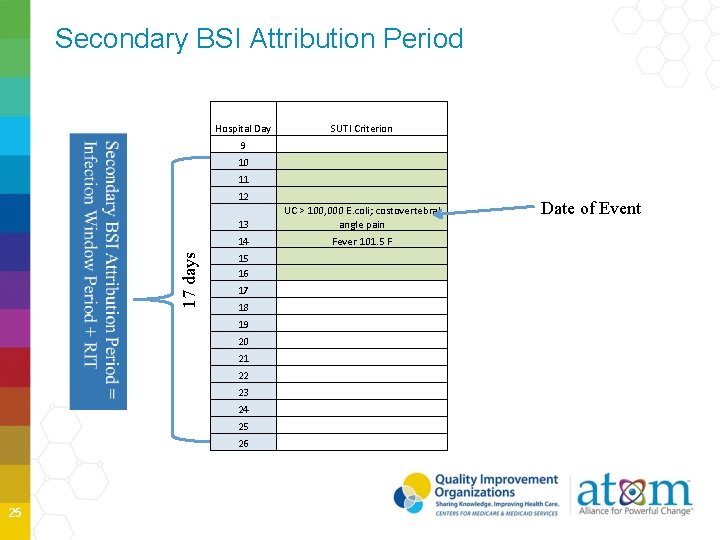
17 days Secondary BSI Attribution Period 25 Hospital Day SUTI Criterion 9 10 11 12 13 UC > 100, 000 E. coli; costovertebral angle pain 14 Fever 101. 5 F 15 16 17 18 19 20 21 22 23 24 25 26 Date of Event

Temperature Changes for 2015 No longer require core temperatures Used documented temperature for all surveillance Do not convert based on site 26

Secondary Bloodstream Infection (BSI) 2014 No objective time period for associating BSI to another infection 27 2015 Secondary BSI Attribution Period

Secondary BSI Attribution Period in which a positive blood culture must be collected to be considered as a secondary bloodstream infection to infection at another primary site Period includes the Infection Window Period combined with the Repeat Timeframe (RIT) Period is 14 -17 days in length depending on the date of event Primary BSIs do not have a Secondary BSI Attribution Period 28

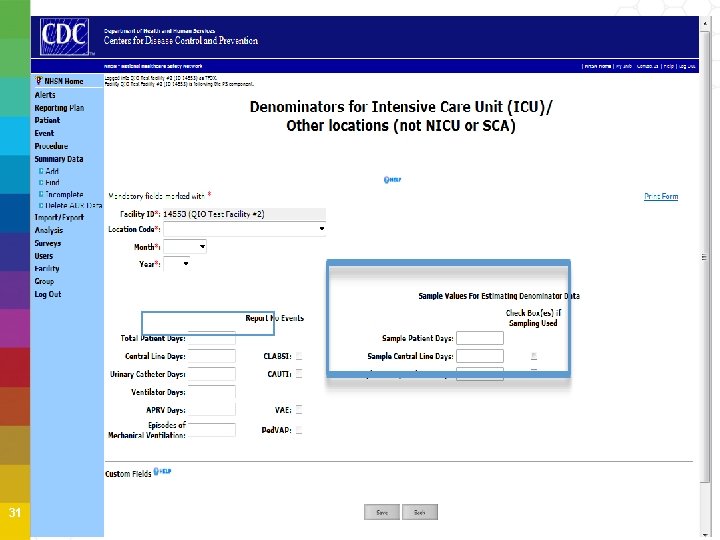
Device-Associated Denominator Sampling Beginning January 2015, hospitals can begin to use an alternative method of collecting CLABSI and CAUTI denominator data in eligible ICU and Ward locations • Reduces staff time in manual collection • Requires data collection on the number of patient days, central lines and/or catheter days on a SINGLE DAY once per week • Requires the number of patient days for every day of month Entry of monthly sampling of device days and NHSN will automatically calculate and use the estimated CLABSI and CAUTI denominator 29

Denominator Sampling Estimate denominator data, only for non-oncology ICUs and wards with ≥ 75 device days/month Review each location’s prior year (12 months) to help determine which units or wards are eligible for sampling More information available in the 2015 NHSN Manual 30

31

Updated Protocols and Clarifications Protocols and outline of recent clarifications and UTI and SSI protocol modifications were posted to CDC/NHSN website in early April, 2015 Footers for each updated protocol will have a revision date of April 2015 UTI and SSI Surveillance will be impacted by revisions • SSI modification should be used beginning January 1, 2015 • 2015 changes to the Inpatient and Outpatient OR procedure definition • UTI modification should be used beginning April 1, 2015 • No requirement to edit CAUTI data for 1 st quarter, 2015 32

Food for Thought What admission and discharge date should be entered when the patient is admitted into a separately licensed CMS inpatient rehab facility (IRF) that is located inside of my acute hospital? For NHSN purposes, if the IRF is set up as a patient care location within Hospital, movement between the acute care hospital and the IRF location should not be counted as a separate facility discharge and admission. Movements should be considered location transfers and counted as one Admission and one discharge from the acute care hospital. 33

Summary 2015 Surveillance definitions for specific infection types • BRON no longer an NHSN infection • New CDI Infection • Other important changes Temperatures are as documented in the chart New alternative device day count option- weekly • Available for certain locations • Minimal average device days must be ≥ 75/month in prior year 34

Summary 2015 (cont’d) GAP DAY concept no longer used Date of Event- first date of element during infection window period POA vs. HAI definition unchanged Secondary BSI Attribution Period • Time-limited Secondary BSI Rules • Simplified- blood culture matching site or part of infection definition Pathogen assignment • Add on if in RIT and not an excluded organism • Organism may be added to more than one event 35

Lab. ID Event Reporting 36

MRSA Bacteremia & C. difficile Reporting MRSA: S. aureus testing oxacillin, cefoxition, or methicillin resistant; or positive from molecular testing for mec. A and PBP 2 a C. difficile: positive result for laboratory test for C. difficile toxin A and/or B or toxin-producing C. difficile detected in stool specimen by culture or by nucleic acid amplification testing by polymerase-chain reaction or PCR • Testing should be performed ONLY on unformed stool specimens 37

MRSA Bacteremia & Clostridium difficile Reporting for 2015 Participating in CMS Inpatient Quality Reporting (IQR) Program Acute Hospitals must report MRSA Bacteremia and C. difficile LABID Events at Facility-Wide Inpatient Level (Fac. Wide. IN) 38

Fac. Wide. IN Reporting Facility-wide Inpatient Fac. Wide. IN • Includes inpatient locations • Includes observation patients housed in an inpatient location • All specimens or blood specimens only from each outpatient emergency department and 24 -hour observation location lood B y l n o , s n cime e p s l l a g n i S eport M r C s i h t y i t i w l i d c If fa share e b l l i w a t da Specimen 39

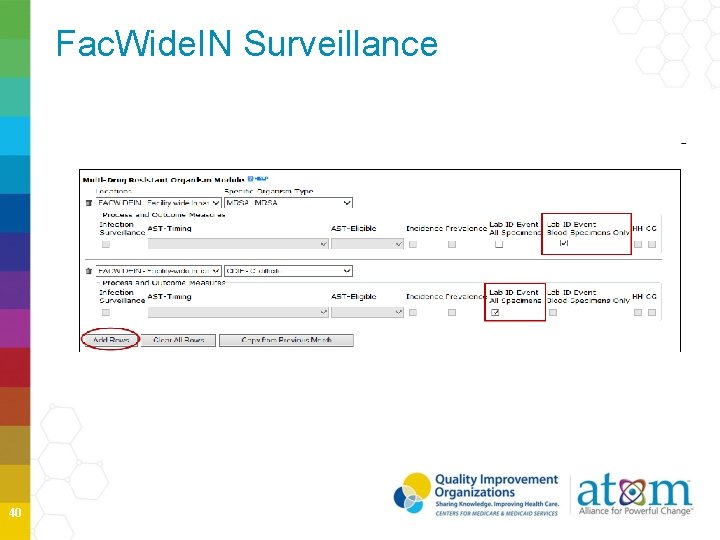
Fac. Wide. IN Surveillance 40

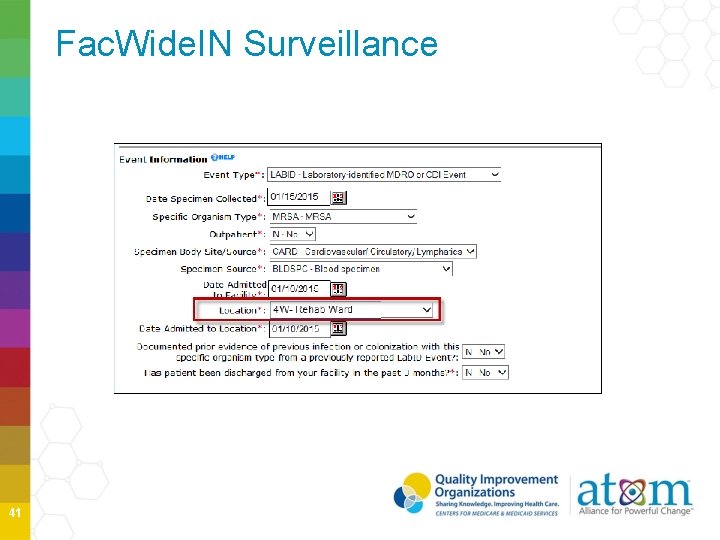
Fac. Wide. IN Surveillance 41

Fac. Wide. IN Surveillance 42

What facility admission date should be used? Acute Care Hospitals and CMS-IRF • The admission date should reflect the date the patient was physically admitted to the inpatient unit in the hospital 43

Lab. ID Events Checklist Review location options and map locations in NHSN Review Monthly Reporting Plan and update as needed Identify and enter all MRSA bacteremia and C. difficile Lab. ID events into NHSN by LOCATION using the MDRO/CDI Lab. ID Event protocol Enter denominator data for each month under surveillance Resolve ”ALERTS” 44

Lab. ID Events Summary Must be reported and monitored throughout all inpatient locations within the facility (except for locations traditionally house predominantly newborns) Location specific reporting is required for CMS-IRF Specimens and Lab. ID Events collected from ED and 24 -hour observation must be reported for outpatient locations regardless of whether patient is later admitted or not Denominator counts are reported separately for each outpatient location Specimens collected from other affiliated outpatient locations may be entered for Fac. Wide. IN ONLY if specimen collection date and admission date are the same 45

CAUTI Reporting 46

Consider for CAUTI Surveillance All CAUTIs require a positive urine culture. Know you laboratory’s urine culture policies: • Ranges for CFU reported • Positive urine cultures are reported for the unit where they were collected • Minimal CFUs are reported 47

CMS Hospital Inpatient Quality Reporting Program 2015 Acute Care Hospitals Unless a “Hospital IQR Program Healthcare-Associated Infection (HAI) Exception Form” is submitted 2015 added adult and pediatric medical, surgical and medical/surgical wards to all ICUs except NICUs 48

Urinary Tract Infection Definitions Two Types of UTIs Symptomatic UTI- SUTI Asymptomatic Bacteremic UTI-ABUTI d e t r po e r e ng b t s orti u m rep , d te ired a i oc equ s s r a I r e T t e U h t A a C c if MS , s pe the C y t th t of o B par as 49

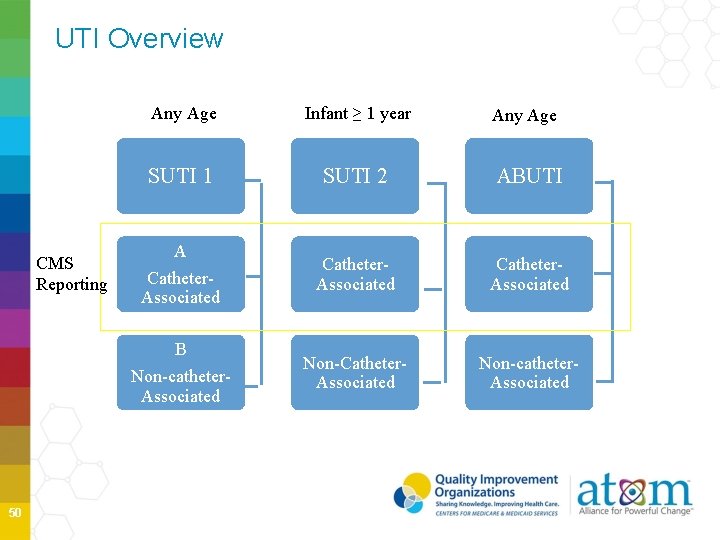
UTI Overview CMS Reporting Any Age Infant ≥ 1 year Any Age SUTI 1 SUTI 2 ABUTI Catheter. Associated Non-Catheter. Associated Non-catheter. Associated A Catheter. Associated B Non-catheter. Associated 50

SUTI Definition Symptoms of a true UTI will vary depending on whether or not a device is present CANNOT use the following symptoms to determine an UTI in a catheterized patient for NHSN: • Frequency • Urgency • Dysuria 51 Infants will exhibit symptoms differently from other ages For infants the following additional symptoms may be used • Apnea • Bradycardia • Lethargy • Vomiting • Hypothermia < 36. 0 C

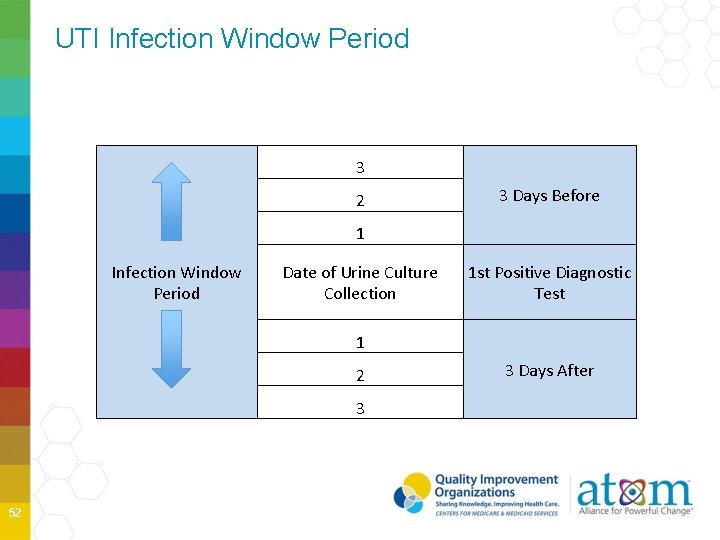
UTI Infection Window Period 3 2 3 Days Before 1 Infection Window Period Date of Urine Culture Collection 1 st Positive Diagnostic Test 1 2 3 52 3 Days After

SUTI 1 a (catheter in place) Patient must meet 1, 2 and 3 below 1. Patient has an indwelling urinary catheter in place for the entire day on the date of event and such catheter has been in place >2 calendar days, on the that date 2. Patient has at least one of the following signs or symptoms • Fever (> 38. 0˚C) or • Suprapubic tenderness • Costovertebral angle pain or tenderness 3. Patient has a UC with no more than two species of organisms, and at least one of which has a colony count of ≥ 105 CFU/ml and all elements of UTI criterion occurs during the Infection Window Period 53

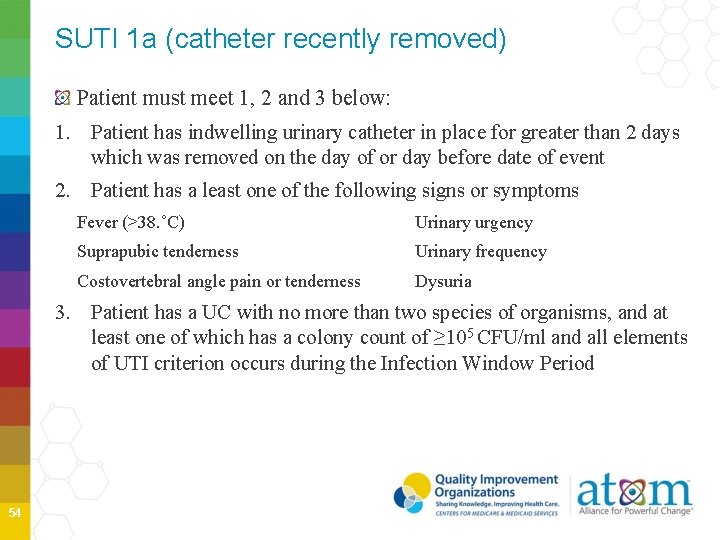
SUTI 1 a (catheter recently removed) Patient must meet 1, 2 and 3 below: 1. Patient has indwelling urinary catheter in place for greater than 2 days which was removed on the day of or day before date of event 2. Patient has a least one of the following signs or symptoms Fever (>38. ˚C) Urinary urgency Suprapubic tenderness Urinary frequency Costovertebral angle pain or tenderness Dysuria 3. Patient has a UC with no more than two species of organisms, and at least one of which has a colony count of ≥ 105 CFU/ml and all elements of UTI criterion occurs during the Infection Window Period 54

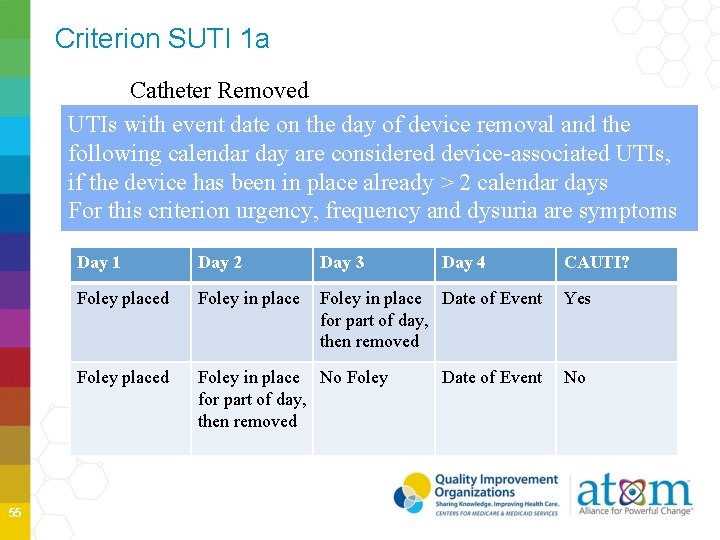
Criterion SUTI 1 a Catheter Removed UTIs with event date on the day of device removal and the following calendar day are considered device-associated UTIs, if the device has been in place already > 2 calendar days For this criterion urgency, frequency and dysuria are symptoms 55 Day 1 Day 2 Day 3 Day 4 Foley placed Foley in place Date of Event for part of day, then removed Foley placed Foley in place No Foley for part of day, then removed Date of Event CAUTI? Yes No

SUTI 1 b (Non-catheter-associated) Patient must meet 1, 2, and 3 below: 1. One of the following is true: • Patient has/had an indwelling catheter but it has/had not been in place> 2 calendar days OR • Patient did not have a urinary catheter in place on the date of event or the day before the date of event 2. Patient has at least ONE of the following S&S Fever (>38. ˚C) Urinary urgency Suprapubic tenderness Urinary frequency Costovertebral angle pain or tenderness Dysuria 3. Patient has a UC with no more than two species of organisms, and at least one of which has a colony count of ≥ 105 CFU/ml and all elements of UTI criterion occurs during the Infection Window Period 56

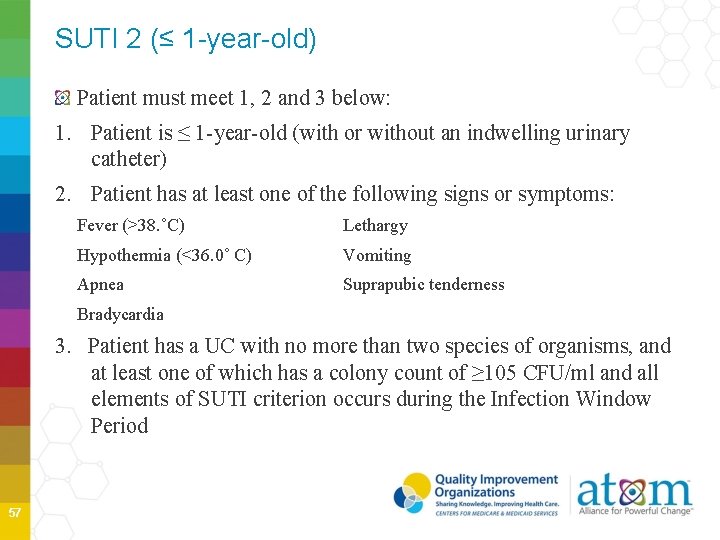
SUTI 2 (≤ 1 -year-old) Patient must meet 1, 2 and 3 below: 1. Patient is ≤ 1 -year-old (with or without an indwelling urinary catheter) 2. Patient has at least one of the following signs or symptoms: Fever (>38. ˚C) Lethargy Hypothermia (<36. 0˚ C) Vomiting Apnea Suprapubic tenderness Bradycardia 3. Patient has a UC with no more than two species of organisms, and at least one of which has a colony count of ≥ 105 CFU/ml and all elements of SUTI criterion occurs during the Infection Window Period 57

No more than 2 Species of Bacteria UC with >2 organisms are not used for NHSN definition “Mixed Flora” or equivalent cannot be used to meet definition Organisms of the same genus but different species = 2 organisms Same organism with different antimicrobial susceptibilities = 1 organism Ex. MRSA = MSSA 58

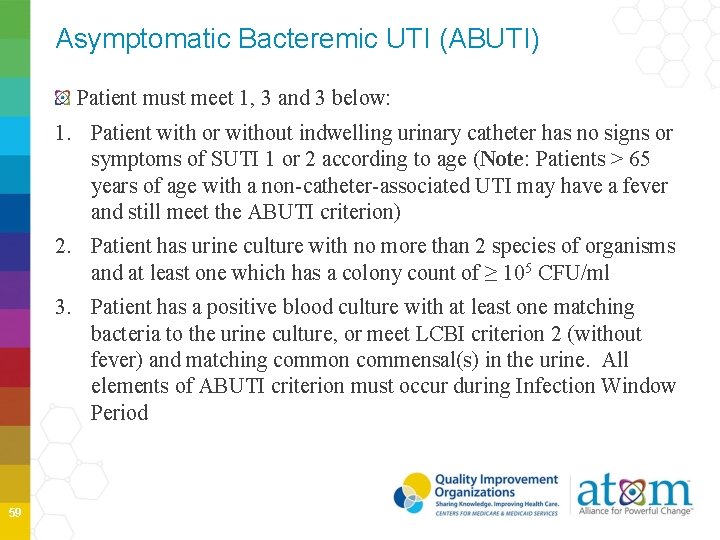
Asymptomatic Bacteremic UTI (ABUTI) Patient must meet 1, 3 and 3 below: 1. Patient with or without indwelling urinary catheter has no signs or symptoms of SUTI 1 or 2 according to age (Note: Patients > 65 years of age with a non-catheter-associated UTI may have a fever and still meet the ABUTI criterion) 2. Patient has urine culture with no more than 2 species of organisms and at least one which has a colony count of ≥ 105 CFU/ml 3. Patient has a positive blood culture with at least one matching bacteria to the urine culture, or meet LCBI criterion 2 (without fever) and matching common commensal(s) in the urine. All elements of ABUTI criterion must occur during Infection Window Period 59

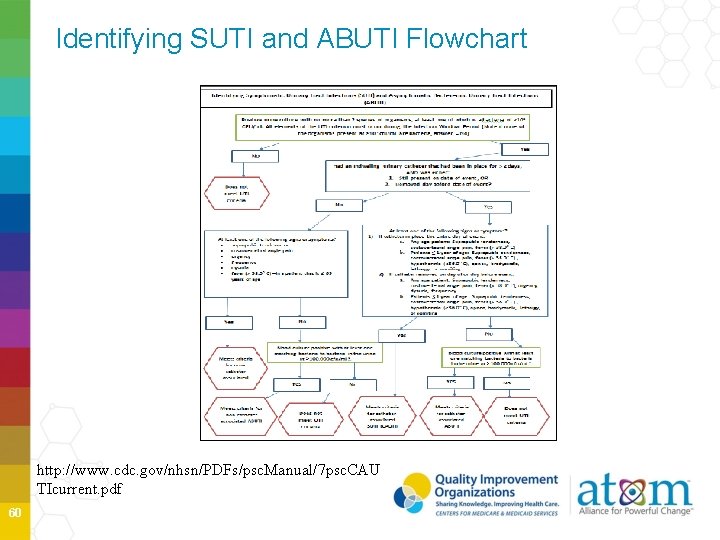
Identifying SUTI and ABUTI Flowchart http: //www. cdc. gov/nhsn/PDFs/psc. Manual/7 psc. CAU TIcurrent. pdf 60

No other Recognized Cause Fever and hypothermia are non-specific symptoms of infection and cannot be excluded from UTI determination because they are clinically deemed due to another recognized cause. 61 http: //phil. cdc. gov/phil/advancedsearchresults. asp

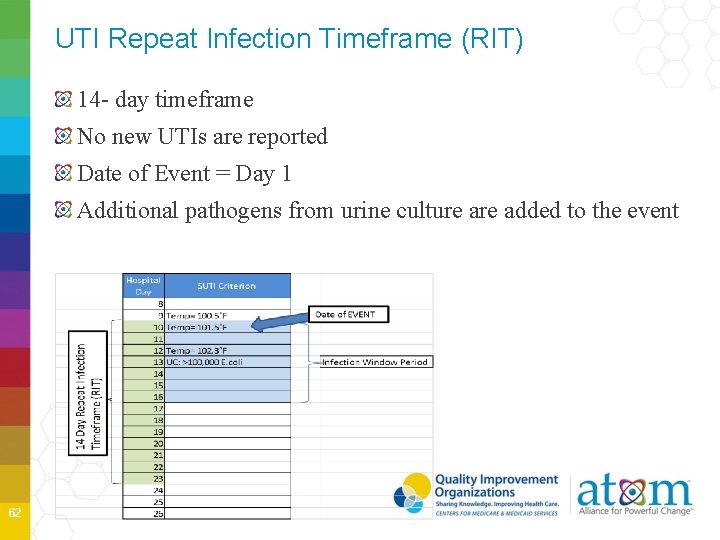
UTI Repeat Infection Timeframe (RIT) 14 - day timeframe No new UTIs are reported Date of Event = Day 1 Additional pathogens from urine culture added to the event 62

Applying CAUTI RIT Patient is admitted to 5 W on 1/15/2015 with a positive UC >100, 000 of E. coli. No S&S present. Foley is inserted on admission. 7 days later (1/22/15), patient has fever (38. 5˚C) and is culture positive from E. coli, >100, 000. Is this a POA? • No, did not meet criteria of POA on admission Is this an HAI? • Yes, what type? CAUTI, Event Date of 1/22/15 63

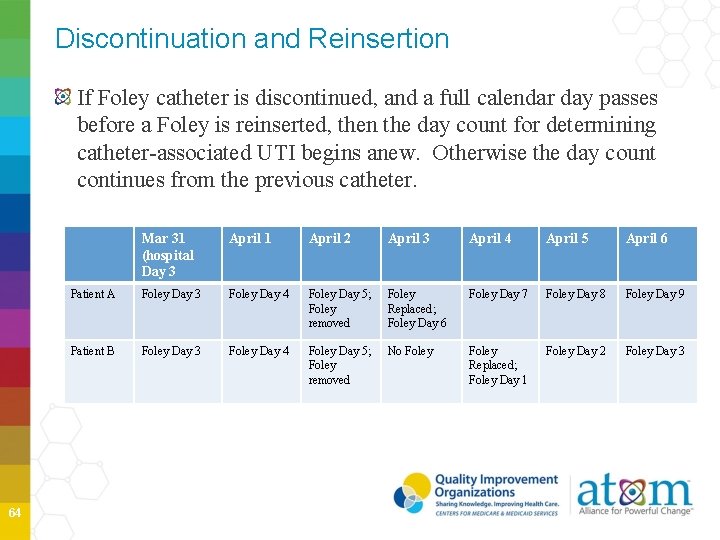
Discontinuation and Reinsertion If Foley catheter is discontinued, and a full calendar day passes before a Foley is reinserted, then the day count for determining catheter-associated UTI begins anew. Otherwise the day count continues from the previous catheter. 64 Mar 31 (hospital Day 3 April 1 April 2 April 3 April 4 April 5 April 6 Patient A Foley Day 3 Foley Day 4 Foley Day 5; Foley removed Foley Replaced; Foley Day 6 Foley Day 7 Foley Day 8 Foley Day 9 Patient B Foley Day 3 Foley Day 4 Foley Day 5; Foley removed No Foley Replaced; Foley Day 1 Foley Day 2 Foley Day 3

Location of Attribution The location where the patient was assigned on the date of the UTI Event, which is further defined as the date that the first element used to meet the UTI infection criterion occurred for the first time in the Infection Window Period Exception - Transfer Rule • If the date of the UTI event is the day of transfer or the next day, the UTI is attributed to transferring location or facility. Likewise, if the date of event is the day of discharge or the next day, the infection is attributed to the discharging location Receiving facility should share information about HAIs with the transferring location or facility to enable complete reporting 65

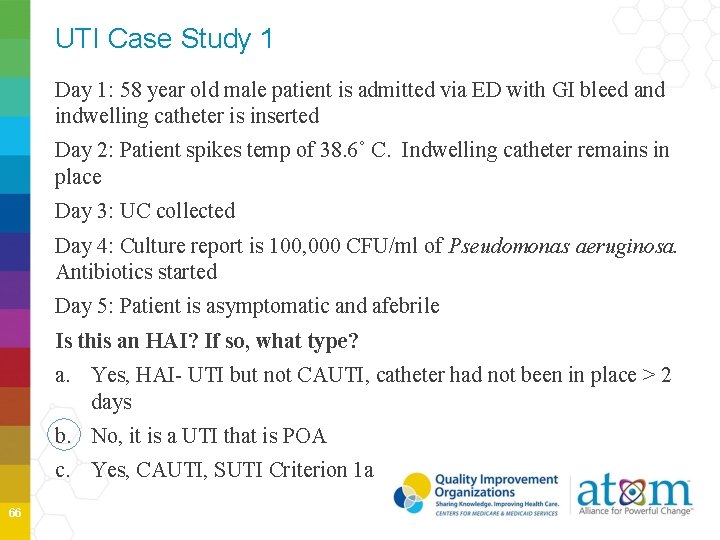
UTI Case Study 1 Day 1: 58 year old male patient is admitted via ED with GI bleed and indwelling catheter is inserted Day 2: Patient spikes temp of 38. 6˚ C. Indwelling catheter remains in place Day 3: UC collected Day 4: Culture report is 100, 000 CFU/ml of Pseudomonas aeruginosa. Antibiotics started Day 5: Patient is asymptomatic and afebrile Is this an HAI? If so, what type? a. Yes, HAI- UTI but not CAUTI, catheter had not been in place > 2 days b. No, it is a UTI that is POA c. Yes, CAUTI, SUTI Criterion 1 a 66

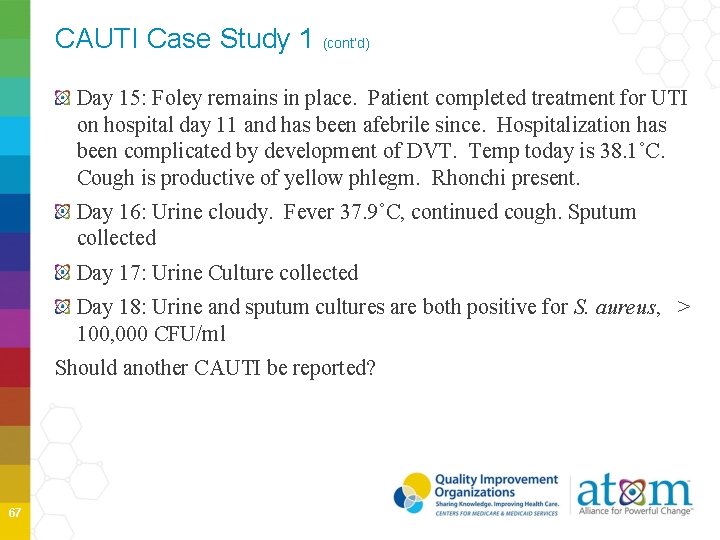
CAUTI Case Study 1 (cont’d) Day 15: Foley remains in place. Patient completed treatment for UTI on hospital day 11 and has been afebrile since. Hospitalization has been complicated by development of DVT. Temp today is 38. 1˚C. Cough is productive of yellow phlegm. Rhonchi present. Day 16: Urine cloudy. Fever 37. 9˚C, continued cough. Sputum collected Day 17: Urine Culture collected Day 18: Urine and sputum cultures are both positive for S. aureus, > 100, 000 CFU/ml Should another CAUTI be reported? 67

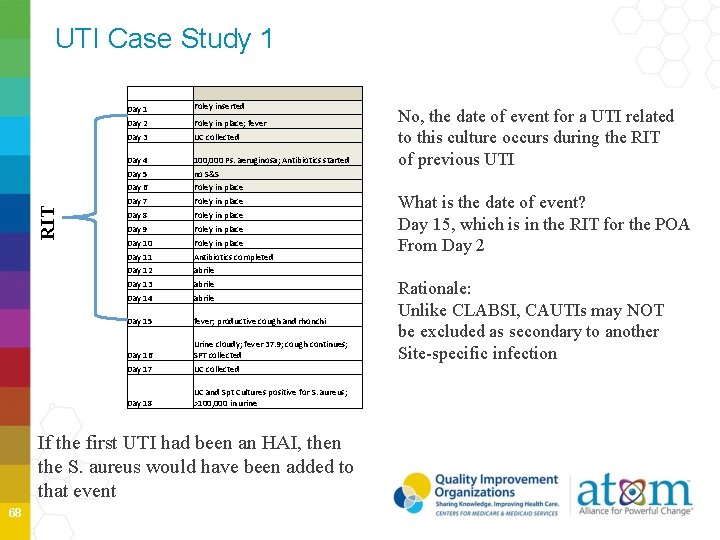
UTI Case Study 1 RIT Day 1 Foley inserted Day 2 Foley in place; fever Day 3 UC collected Day 4 100, 000 Ps. aeruginosa; Antibiotics started Day 5 no S&S Day 6 Foley in place Day 7 Foley in place Day 8 Foley in place Day 9 Foley in place Day 10 Foley in place Day 11 Antibiotics completed Day 12 abrile Day 13 abrile Day 14 abrile Day 15 fever; productive cough and rhonchi Day 16 Urine cloudy; fever 37. 9; cough continues; SPT collected Day 17 UC collected Day 18 UC and Spt Cultures positive for S. aureus; >100, 000 in urine If the first UTI had been an HAI, then the S. aureus would have been added to that event 68 No, the date of event for a UTI related to this culture occurs during the RIT of previous UTI What is the date of event? Day 15, which is in the RIT for the POA From Day 2 Rationale: Unlike CLABSI, CAUTIs may NOT be excluded as secondary to another Site-specific infection

CAUTI Summary All CAUTIs require a positive urine culture Minimal CFU/ml > 100, 000 of no more than 2 species Account for positive UC from the ED which may represent recently discharged patients Infection Window Period is a 7 -day period (3 days before positive diagnostic test and 3 day after) RIT period uses the date of event to determine a 14 -day timeframe during which no new infections of the same type are reported. Date of event is Day 1 of the timeframe. If date of event of subsequent infection is within the 14 day period, additional organisms are added to the original event. 69

Thank You Slides and information adapted from CDC/NHSN Training slides and materials 70