2013 Lymphoma Update 2013 08 05 Outline Follicular



























- Slides: 51

2013 Lymphoma Update 2013. 08. 05

Outline • Follicular lymphoma • Hodgkin’s lymphoma • Chronic lymphocytic leukemia

Follicular Lymphoma

Epidemiology of FL • • Account 22% of NHL Chronic relapsing and remitting pattern Most patients aged > 50 Median survival 12~14 years

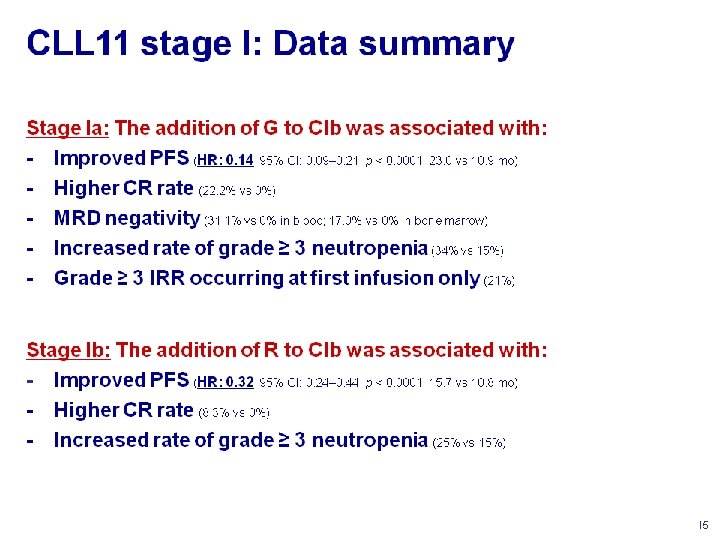
1 st line treatment in FL R-CHOP vs CHOP R-CVP vs CVP 2 -yr OS 95% vs 90% (P=0. 016) 4 -yr OS 83% vs 77% (P=0. 0290) Blood 2005; 106: 3725 Lancet 2013; 381: 1203

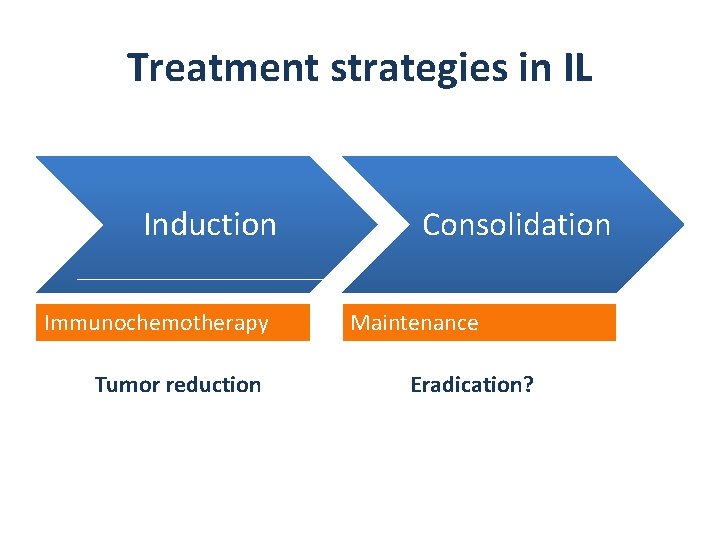
Standard care with indolent lymphoma • There’s still a role for watch& wait, despite new therapy modalities • Combined immuno-chemotherapy is standard of care • Rituximab maintenance as consolidation New perspectives • Which chemotherapy should be best combined with Rituximab?

B-R vs R-CHOP Sti. L NHL 1 -2003 Follicular Waldenstroms Marginal zone Mantle cell Small lymphocytic (n=549) Bendamustine-Rituximab (B: 90 ng/m 2 day 1+2, max 6 cycles, q 4 wks) R 81 centers in Germany Enrolled between Sep 2003~Aug 2008 Stage III/IV IL or MCL Median f/u 45 mos Non-inferiority study R-CHOP (max 6 cycles, q 3 wks) Primary endpoint: PFS Rummel et al. Lancet 2013; 381: 1203

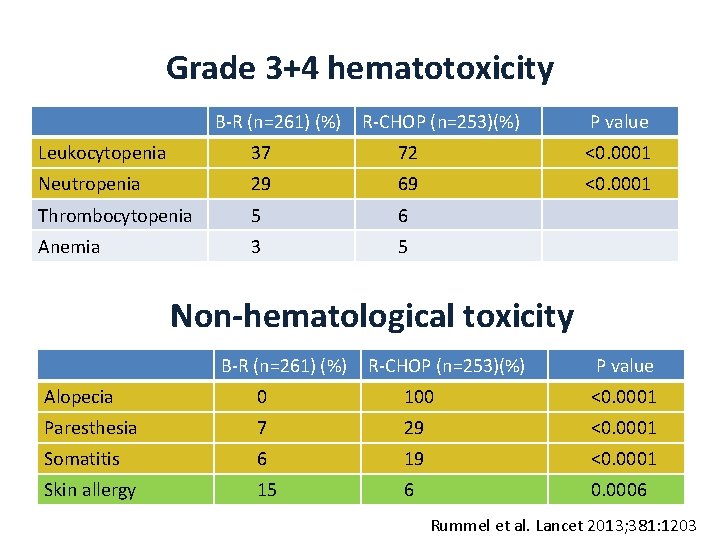
Grade 3+4 hematotoxicity B-R (n=261) (%) R-CHOP (n=253)(%) P value Leukocytopenia 37 72 <0. 0001 Neutropenia 29 69 <0. 0001 Thrombocytopenia 5 6 Anemia 3 5 Non-hematological toxicity B-R (n=261) (%) R-CHOP (n=253)(%) P value Alopecia 0 100 <0. 0001 Paresthesia 7 29 <0. 0001 Somatitis 6 19 <0. 0001 Skin allergy 15 6 0. 0006 Rummel et al. Lancet 2013; 381: 1203

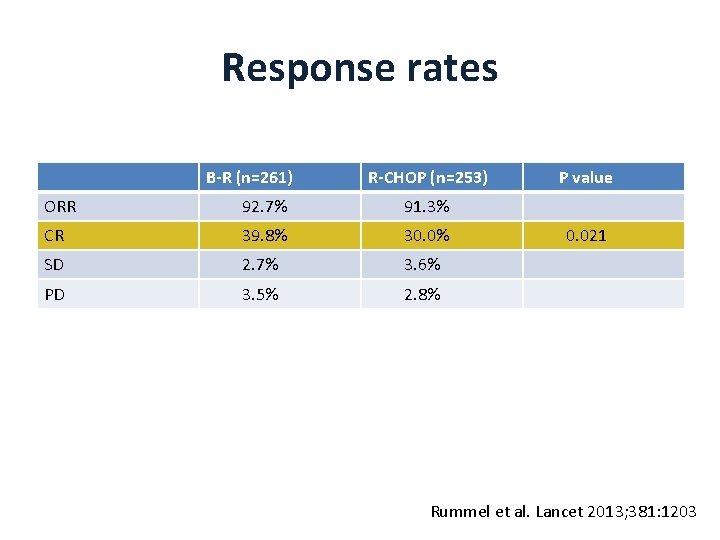
Response rates B-R (n=261) R-CHOP (n=253) ORR 92. 7% 91. 3% CR 39. 8% 30. 0% SD 2. 7% 3. 6% PD 3. 5% 2. 8% P value 0. 021 Rummel et al. Lancet 2013; 381: 1203

PFS B-R R-CHOP P value PFS 69. 5 mos 31. 2 mos P<0. 0001 OS NR NR Rummel et al. Lancet 2013; 381: 1203

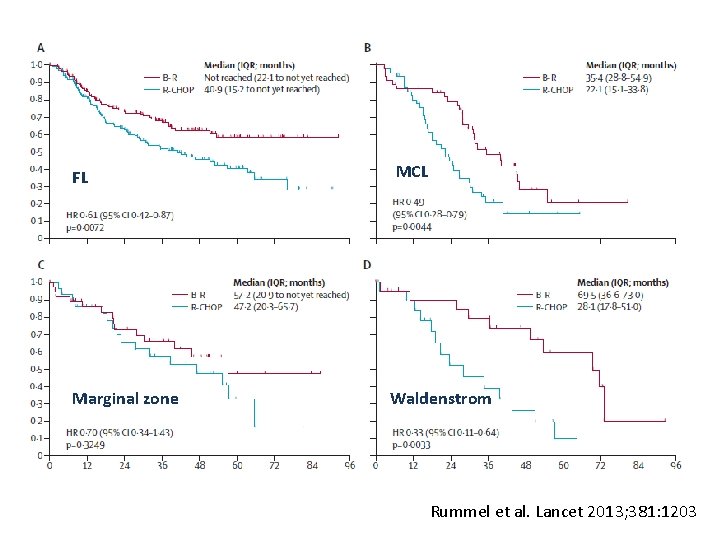
FL Marginal zone MCL Waldenstrom Rummel et al. Lancet 2013; 381: 1203

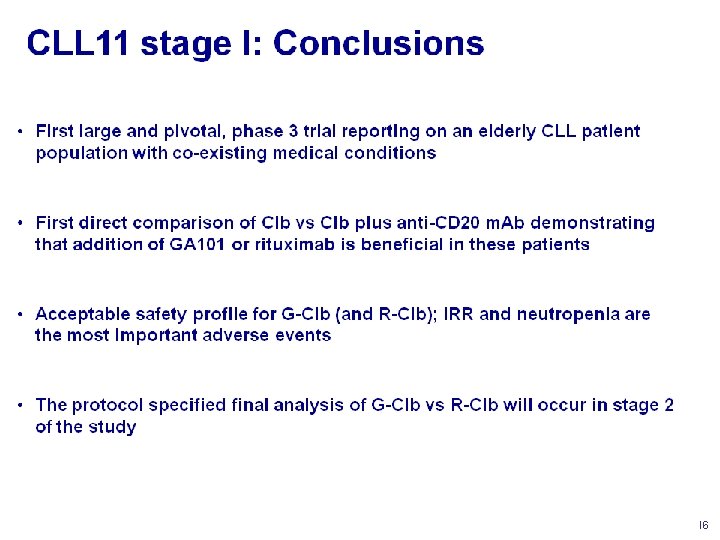
Conclusion • B-R is not only less toxic but also more effective than R-CHOP • B-R could be considered as a preferred 1 st-line treatment for patients with FL, indolent and MCL Rummel et al. Lancet 2013; 381: 1203

Treatment strategies in IL Induction Immunochemotherapy Tumor reduction Consolidation Maintenance Eradication?

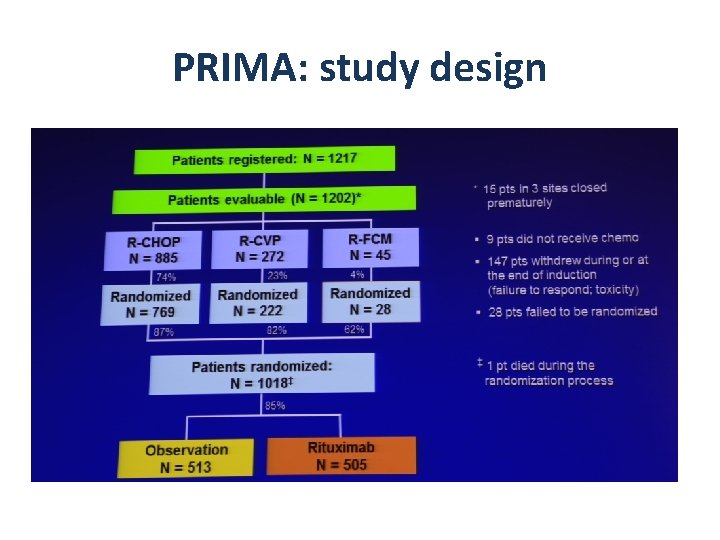
PRIMA: study design

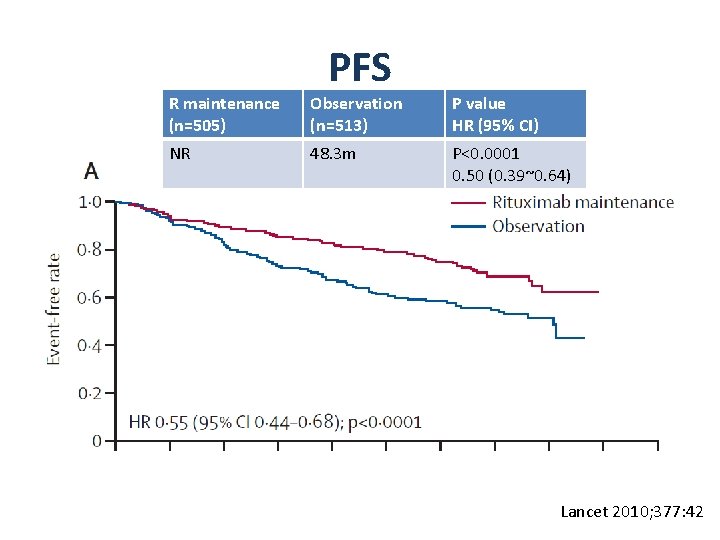
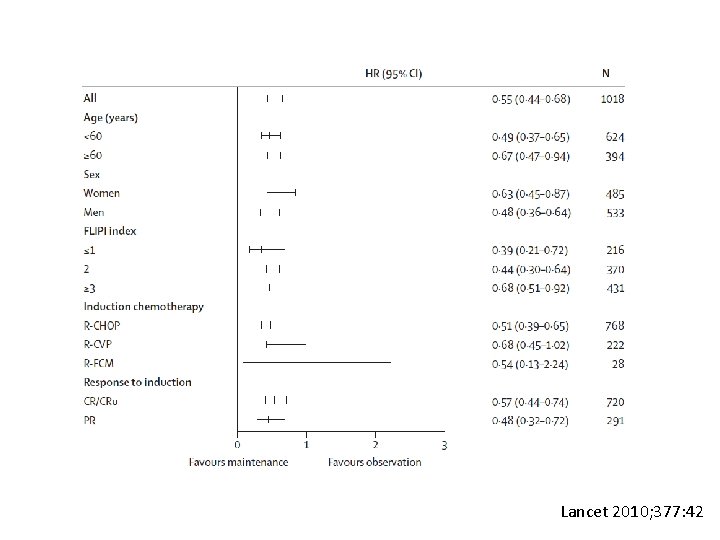
PFS R maintenance (n=505) Observation (n=513) P value HR (95% CI) NR 48. 3 m P<0. 0001 0. 50 (0. 39~0. 64) Lancet 2010; 377: 42

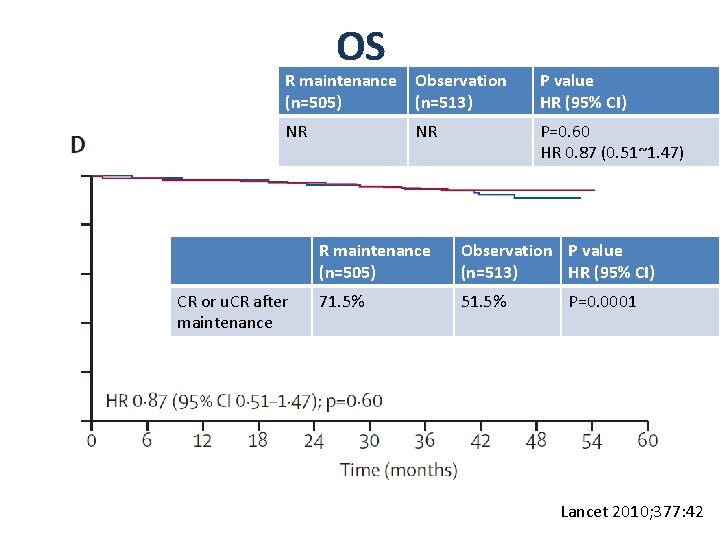
OS R maintenance (n=505) Observation (n=513) P value HR (95% CI) NR NR P=0. 60 HR 0. 87 (0. 51~1. 47) CR or u. CR after maintenance R maintenance (n=505) Observation P value (n=513) HR (95% CI) 71. 5% 51. 5% P=0. 0001 Lancet 2010; 377: 42

Lancet 2010; 377: 42

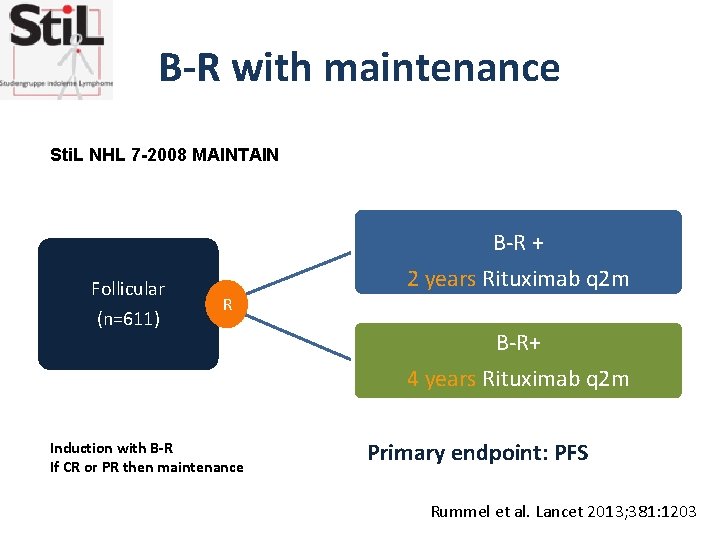
B-R with maintenance Sti. L NHL 7 -2008 MAINTAIN Follicular (n=611) B-R + 2 years Rituximab q 2 m R Induction with B-R If CR or PR then maintenance B-R+ 4 years Rituximab q 2 m Primary endpoint: PFS Rummel et al. Lancet 2013; 381: 1203

Hodgkin’s lymphoma

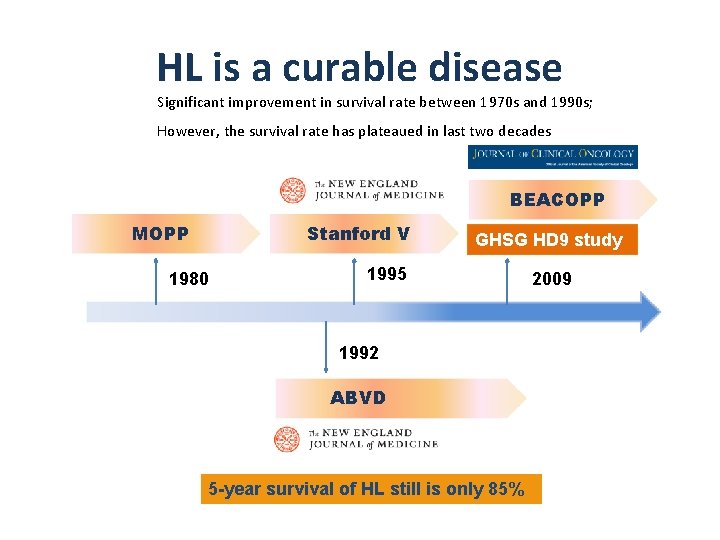
HL is a curable disease Significant improvement in survival rate between 1970 s and 1990 s; However, the survival rate has plateaued in last two decades BEACOPP MOPP Stanford V 1980 GHSG HD 9 study 1995 1992 ABVD 5 -year survival of HL still is only 85% 2009

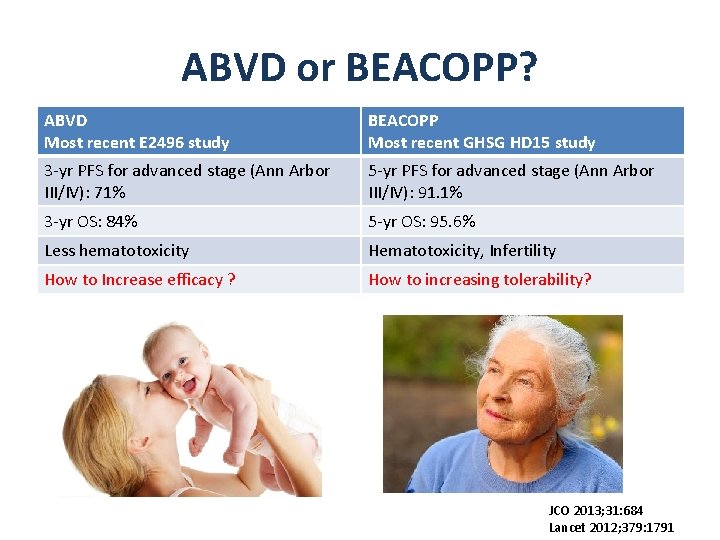
ABVD or BEACOPP? ABVD Most recent E 2496 study BEACOPP Most recent GHSG HD 15 study 3 -yr PFS for advanced stage (Ann Arbor III/IV): 71% 5 -yr PFS for advanced stage (Ann Arbor III/IV): 91. 1% 3 -yr OS: 84% 5 -yr OS: 95. 6% Less hematotoxicity Hematotoxicity, Infertility How to Increase efficacy ? How to increasing tolerability? JCO 2013; 31: 684 Lancet 2012; 379: 1791

Different approaches to targeting CD 30 Anti-CD 30 naked m. Ab CD 30 Anti-CD 30 ADCs Modified anti. CD 30 Ab (improving receptor affinity)

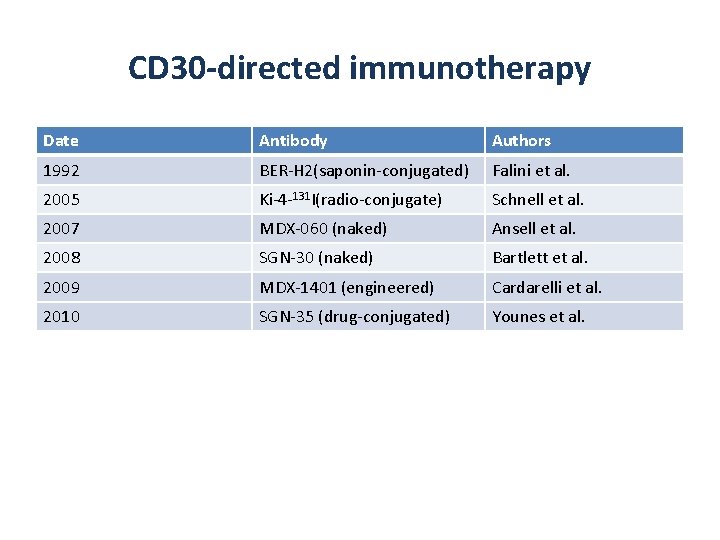
CD 30 -directed immunotherapy Date Antibody Authors 1992 BER-H 2(saponin-conjugated) Falini et al. 2005 Ki-4 -131 I(radio-conjugate) Schnell et al. 2007 MDX-060 (naked) Ansell et al. 2008 SGN-30 (naked) Bartlett et al. 2009 MDX-1401 (engineered) Cardarelli et al. 2010 SGN-35 (drug-conjugated) Younes et al.

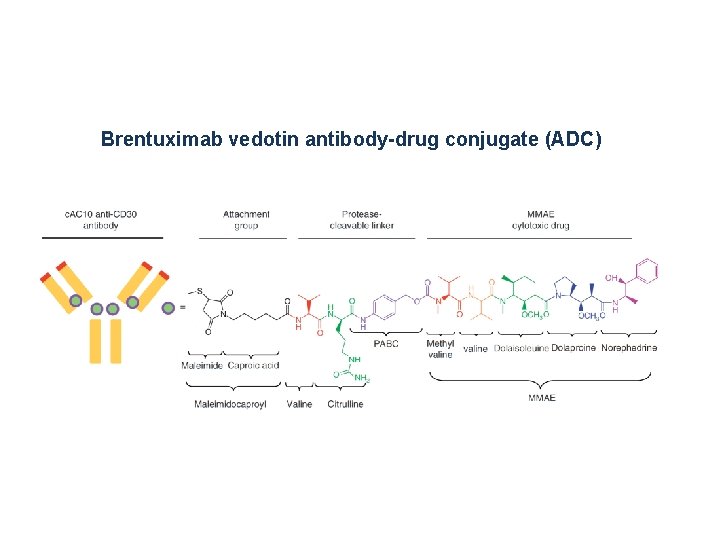
Brentuximab vedotin antibody-drug conjugate (ADC)

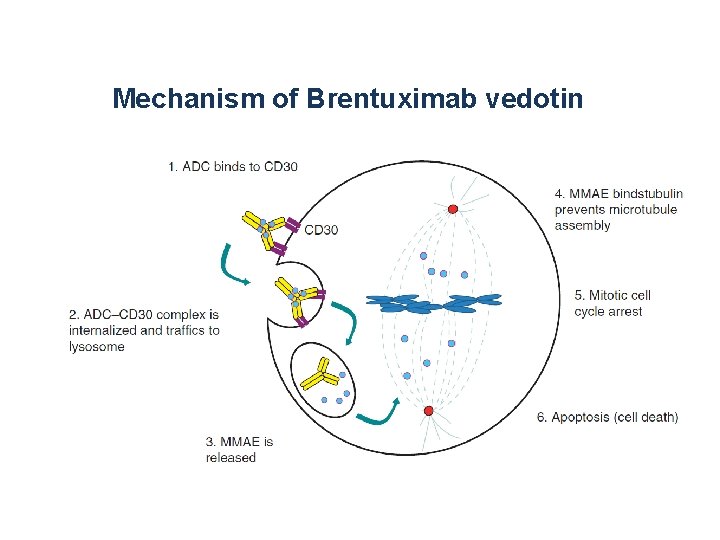
Mechanism of Brentuximab vedotin

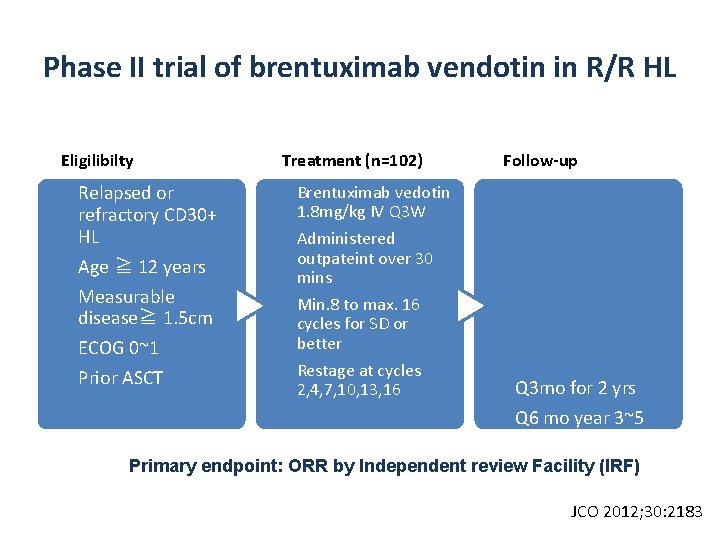
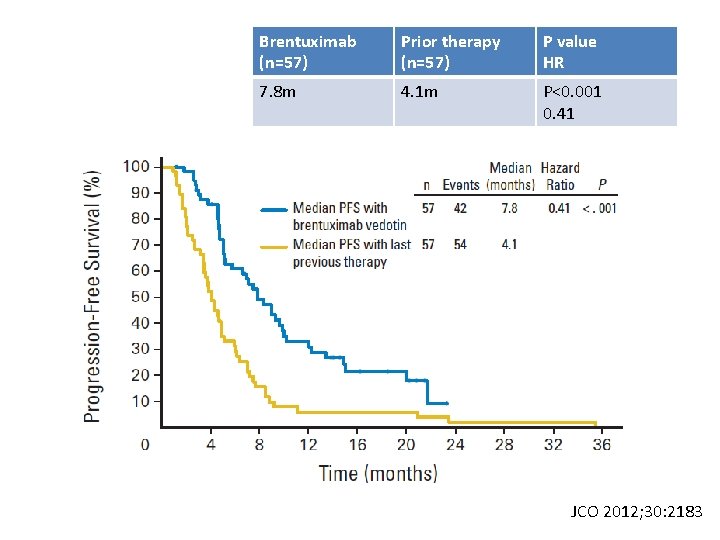
Phase II trial of brentuximab vendotin in R/R HL Eligilibilty Relapsed or refractory CD 30+ HL Age ≧ 12 years Measurable disease≧ 1. 5 cm ECOG 0~1 Prior ASCT Treatment (n=102) Follow-up Brentuximab vedotin 1. 8 mg/kg IV Q 3 W Administered outpateint over 30 mins Min. 8 to max. 16 cycles for SD or better Restage at cycles 2, 4, 7, 10, 13, 16 Q 3 mo for 2 yrs Q 6 mo year 3~5 Annually after 5 Primary endpoint: ORR by Independent review Facility (IRF) years JCO 2012; 30: 2183

IRF (n=102) ORR, % (95% CI) CR, % (95% CI) 75 (65, 83) 34 (25, 44) PR, % 40 SD, % 22 PD, % 3 Not evaluable, % 1 JCO 2012; 30: 2183

Brentuximab (n=57) Prior therapy (n=57) P value HR 7. 8 m 4. 1 m P<0. 001 0. 41 JCO 2012; 30: 2183

Summary: changing therapeutic paradigms? Standard treatment Open questions 3 rd line • Brentuximab vedotin 2 nd line • HDCT+ASCT • DEXA-BEAM, mini-BEAM • ICE, DHAP, GDP Improving salvage? Introducing maintenance? 1 st line • ABVD • BEACOPP New combination?

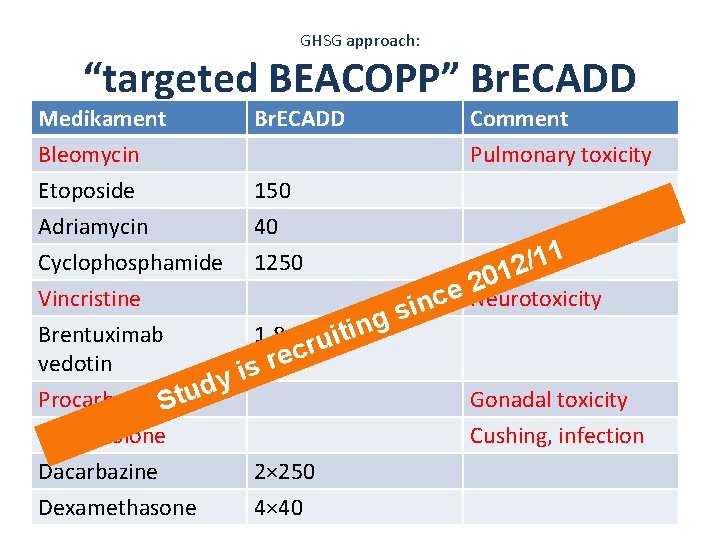
GHSG approach: “targeted BEACOPP” Br. ECADD Medikament Bleomycin Etoposide Adriamycin Br. ECADD 150 40 Comment Pulmonary toxicity 1 1 Cyclophosphamide 1250 / 2 1 0 2 Vincristine ce Neurotoxicity n i s g Brentuximab 1. 8 uitin r c e r vedotin s i y d u Procarbazine. St Gonadal toxicity Prednisolone Cushing, infection Dacarbazine 2× 250 Dexamethasone 4× 40

Chronic lymphocytic leukemia

Classification of CLL patients according to their fitness Blood 2009; 114: 3359


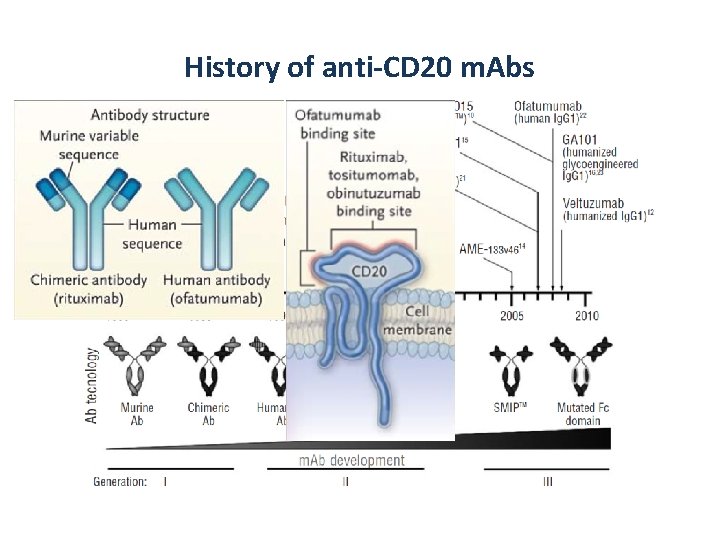
History of anti-CD 20 m. Abs

GA 101: type II, glycoenginered anti-CD 20 m. Ab • First type II, glycoengineered , humanized Ig. G 1 anti-CD 20 m. Ab • In preclinical studies comparing against rituximab, GA 101 provided: ü Enhanced ADCC, oligosaccharides that enhance the interaction with FcγR, particularly FcγRIIIa, even in effector cells bearing the low affinity polymorphic variant of FcγRIIIa ü Increased direct cell death induction ü Decreased complement-dependent cytotoxicity


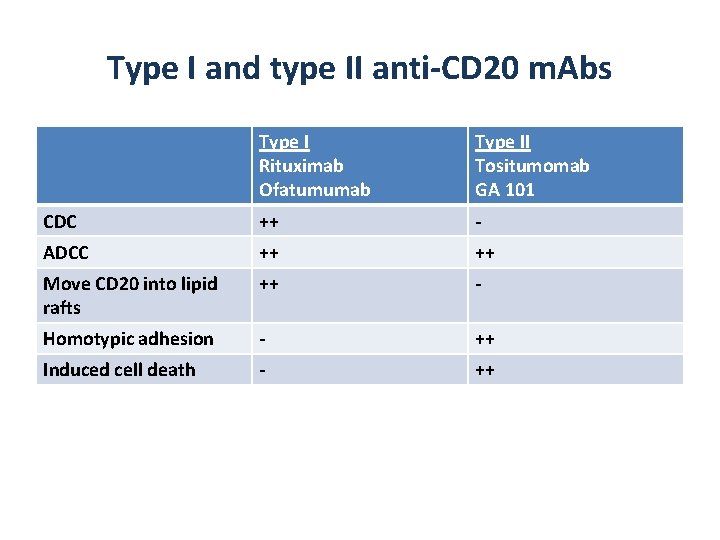
Type I and type II anti-CD 20 m. Abs Type I Rituximab Ofatumumab Type II Tositumomab GA 101 CDC ++ - ADCC ++ ++ Move CD 20 into lipid rafts ++ - Homotypic adhesion - ++ Induced cell death - ++

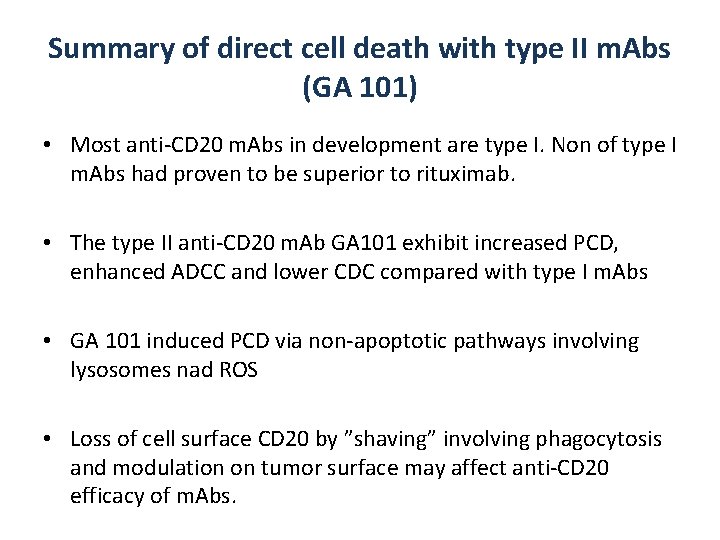
Summary of direct cell death with type II m. Abs (GA 101) • Most anti-CD 20 m. Abs in development are type I. Non of type I m. Abs had proven to be superior to rituximab. • The type II anti-CD 20 m. Ab GA 101 exhibit increased PCD, enhanced ADCC and lower CDC compared with type I m. Abs • GA 101 induced PCD via non-apoptotic pathways involving lysosomes nad ROS • Loss of cell surface CD 20 by ”shaving” involving phagocytosis and modulation on tumor surface may affect anti-CD 20 efficacy of m. Abs.













Thanks for your attention comments and questions
 Sanjana bhagwat
Sanjana bhagwat Alternative of log based recovery
Alternative of log based recovery Follicular epithelium
Follicular epithelium Follicular phase
Follicular phase Follicular cells of thyroid gland
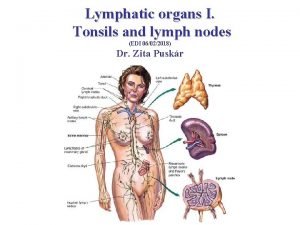
Follicular cells of thyroid gland Lymphatic system organs
Lymphatic system organs Ameloblastoma follicular
Ameloblastoma follicular Follicular carcinoma of thyroid
Follicular carcinoma of thyroid Thyroid cancer
Thyroid cancer Diene stain
Diene stain Enlarged thyroid gland
Enlarged thyroid gland Ovarian follicle
Ovarian follicle Principal cells location
Principal cells location Parafollicular
Parafollicular Oogenesis and follicular development
Oogenesis and follicular development Follicular conjunctivitis
Follicular conjunctivitis Goiter
Goiter Follicular study
Follicular study Hormones
Hormones Thyroglobulin
Thyroglobulin Plot
Plot Burkitt lymphoma cytology
Burkitt lymphoma cytology Ann arbour staging system
Ann arbour staging system Szóbajön
Szóbajön Chylothorax causes
Chylothorax causes Hodgkin's lymphoma clinical presentation
Hodgkin's lymphoma clinical presentation Hepatosplenic t-cell lymphoma
Hepatosplenic t-cell lymphoma Neoplasia
Neoplasia Lymphoma
Lymphoma Luis fayad
Luis fayad Non-hodgkin lymphoma
Non-hodgkin lymphoma Rbac mantle cell lymphoma
Rbac mantle cell lymphoma Ann arbor staging system
Ann arbor staging system Hiv family name
Hiv family name Smoldering lymphoma
Smoldering lymphoma Reed sternberg cells
Reed sternberg cells Hodjkins
Hodjkins Hodgkin's lymphoma classification
Hodgkin's lymphoma classification Reed sternberg cell diagram
Reed sternberg cell diagram Lymphoma case presentation
Lymphoma case presentation Classification of hodgkin lymphoma
Classification of hodgkin lymphoma Lymphoma vs leukemia
Lymphoma vs leukemia Indolent non-hodgkin lymphoma quizlet
Indolent non-hodgkin lymphoma quizlet Bcell lymphoma
Bcell lymphoma Malt lymphoma
Malt lymphoma Burkitt lymphoma
Burkitt lymphoma Pancreazin
Pancreazin Maintenance rituximab mantle cell lymphoma
Maintenance rituximab mantle cell lymphoma Ann arbor staging system
Ann arbor staging system Difference between hodgkin and non hodgkin lymphoma
Difference between hodgkin and non hodgkin lymphoma Sandwich sentence writing
Sandwich sentence writing Siha offline
Siha offline