2013 3 18 1 Classification of Hematologic Malignancies





















- Slides: 62

2013년 3월 18일 1. Classification of Hematologic Malignancies (악성 혈액질환의 분류) 2. Myelodysplastic syndromes (MDS, 골수형성이상증후군) 3. Acute Myeloid Leukemia (AML, 급성골수성백혈병) 4. Myelodysplastic/Myeloproliferative Neoplasms (MDS/MPN 골수형성이상/골수 증식종양)

악성 혈액질환의 분류 Classification of Hematologic Malignancies

Malignancies • 종양 (neoplasm) : – 악성(malignant) --- 양성 (benign) • 악성 종양 (암) : – 고형암(solid) --- 혈액암(hematologic) • 특징 – – Clonality Autonomic proliferation Dysplasia Invasion and metastasis

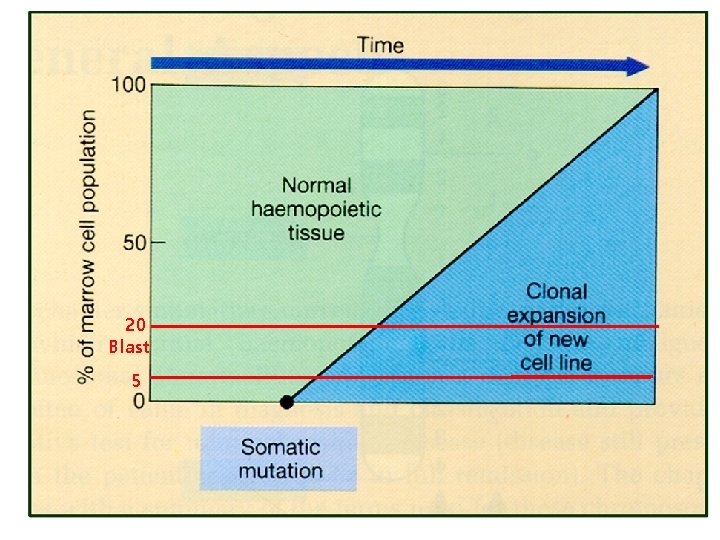
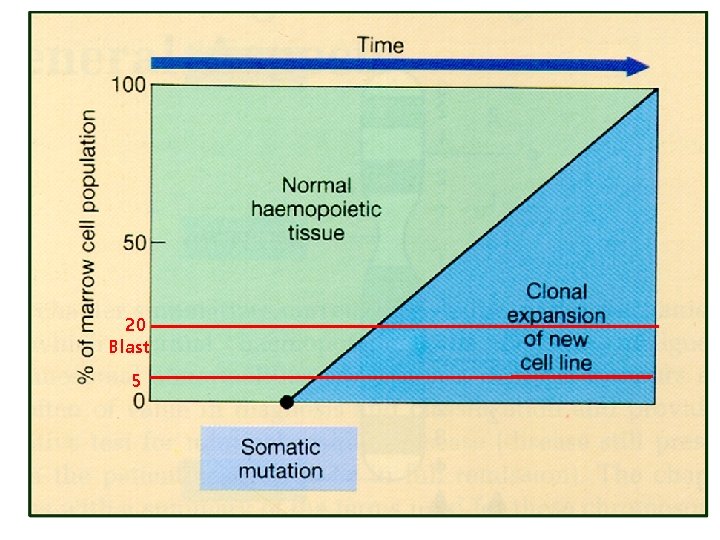
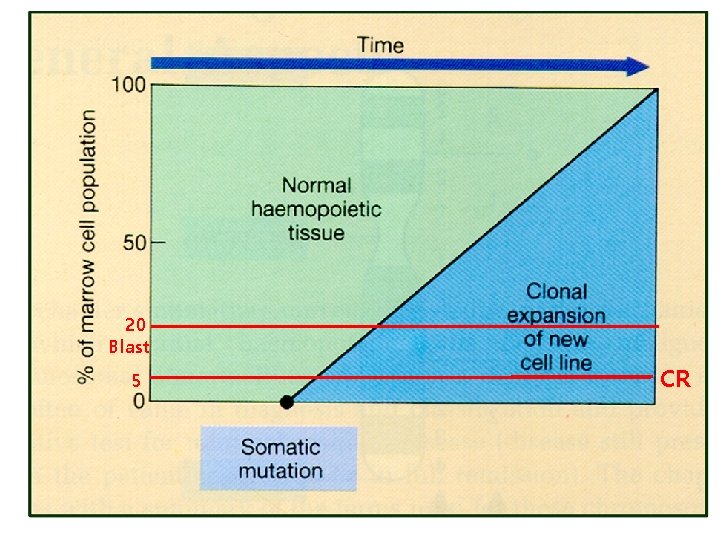
Hematologic Malignancies • Uncontrolled clonal proliferation of hematologic cells : – Origin : stem cells to mature hematologic cells – Differentiation and proliferation 장애 • Hematologic malignancies의 특징 – – Clonality Chronic proliferation Dysplasia Metastasis • Staging – Lymphoma – MM – CLL : MPN : MDS : BM (origin) : Non-TMN : Ann Abor : Durie-Salmon, ISS : Rai, Binet

20 Blast 5

Past classification of hematologic neoplasms • Morphology • Clinical features • Leukemia : – Acute, Chronic ; Myeloid, Lymphoid : AML, CML, ALL, CLL – AML(M 0 -7), ALL(L 1 -3) : • • FAB MDS : RA, RARS, RAEB, CMML : CMPD (MPN) : PV, ET, IMF, CML Lymphoma : HD, NHL : REAL Plasma cell dyscrasia : MGUS, MM, WM

Progress of Classification of Hematologic malignancies • Lymphoma – – – Rappaport classification Kiel classification Lukes-Collins classification Working Formulation Revised European-American classification (REAL) • Leukemia and MDS – French-American-British (FAB) classification • WHO classification 2001 • WHO classification 2008

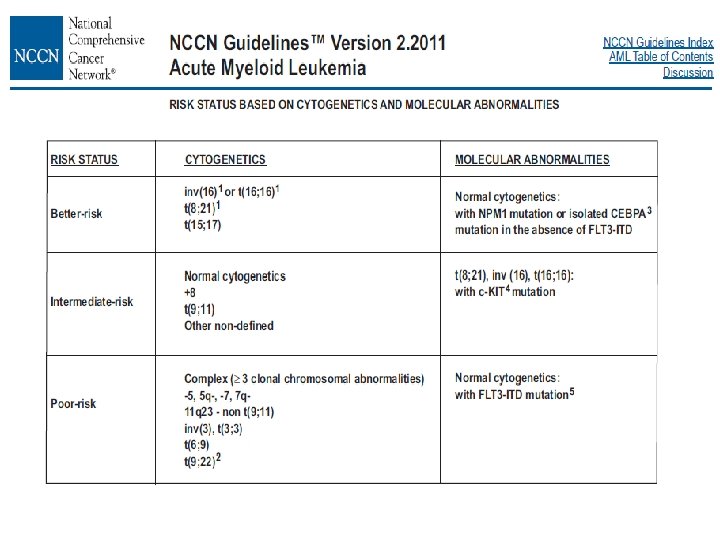
Bases of WHO classification • Morphology : most important part but sometimes not objective • Immunophenotype : lineage, objective, target (ex. CD 20) • Genetic abnormalities : objective – Specific : BCR-ABL, PML-RARA, – Characteristic but not specific : MYC, CCND, BCL 2 rearrangement, JAK 2 mutation – Prognostic : TP 53 mutation or FLT 3 -ITD • Clinical features : age, nodal versus extranodal presentation, specific anatomic site and history of cytotoxic and otherapies • Provisional entities, borderline categories

Stratification according to • Lineages : – myeloid, lymphoid, and histiocytic/dendritic cell – Lineage plasticity – FGFR 1, PDGFA, and PDGFB rearrangement • Maturation : precursor, mature • Biologic features : MPN, MDS • Clinical presentation : mature lymphoid – Disseminated, extranodal, indolent, aggressive – Stage of differentiation • WHO Classification : 2001, 2008,

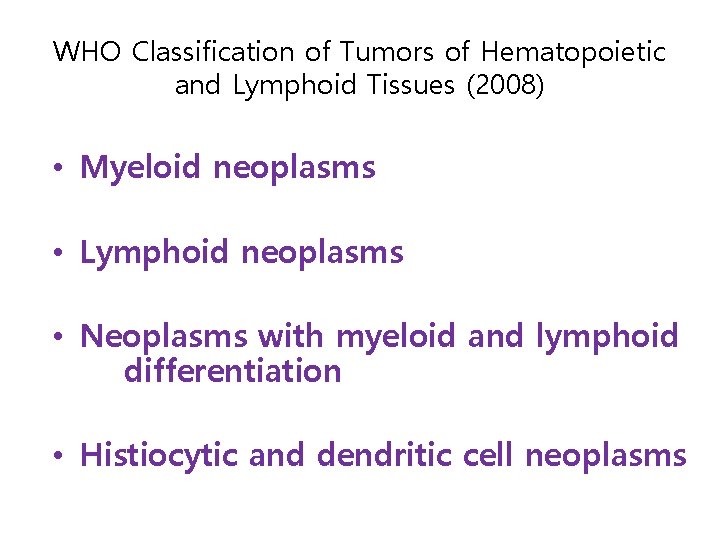
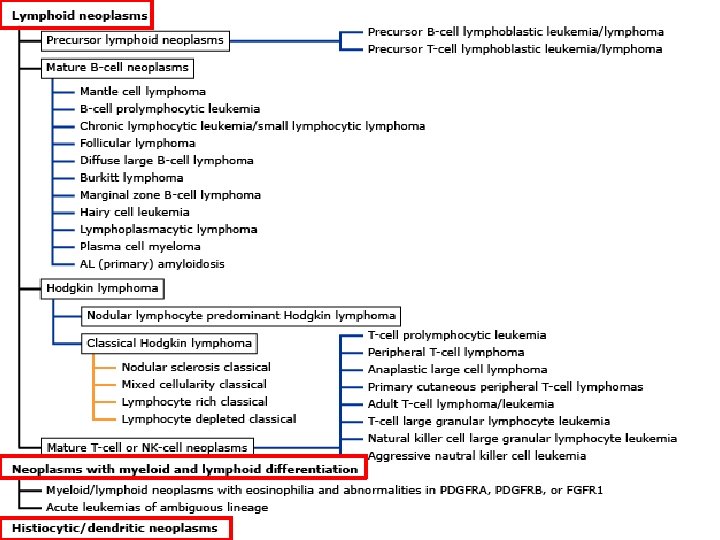
WHO Classification of Tumors of Hematopoietic and Lymphoid Tissues (2008) • Myeloid neoplasms • Lymphoid neoplasms • Neoplasms with myeloid and lymphoid differentiation • Histiocytic and dendritic cell neoplasms




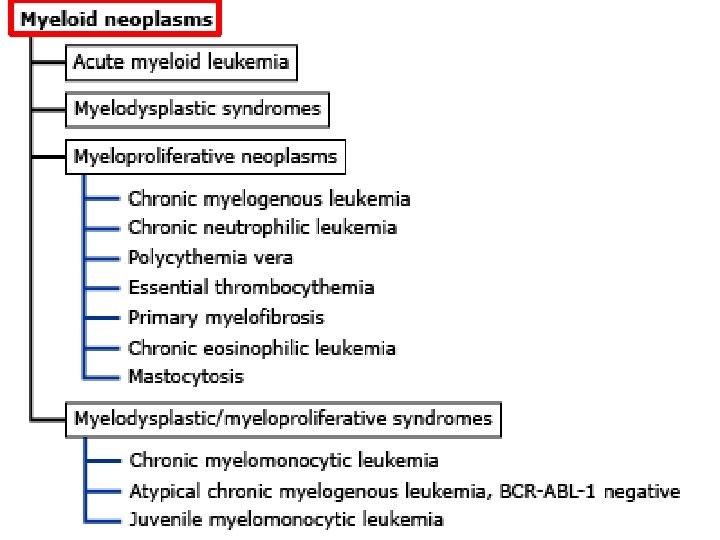
Myeloid neoplasms • Acute myeloid leukemia (AML) and related precursor neoplasms • Myelodysplastic syndrome • Myeloproliferative neoplasms • Myelodysplastic/myeloproliferative neoplasms

20 Blast 5

Myelodysplastic Syndrome (MDS)

MDS 특징 • A heterogenous group of acquired bone marrow disorders • Dysplastic growth of hematopoietic progenitors • Hypercellular bone marrow • Peripheral cytopenia • Propensity to progress to AML

Etiology • Unknown • Chemicals : benzene, pesticides • Chemotherapy and radiation therapy – Therapy-related (t-MDS/AML) • Post ASCT of cancer • Immunotherapy for aplastic anemia

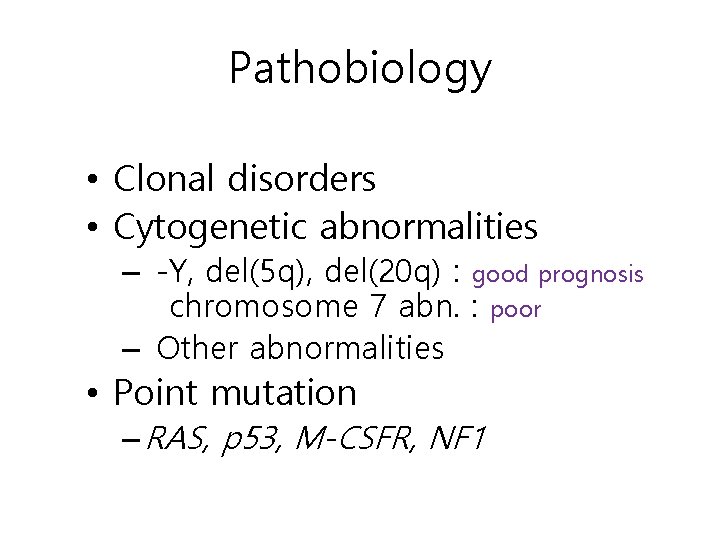
Pathobiology • Clonal disorders • Cytogenetic abnormalities – -Y, del(5 q), del(20 q) : good prognosis chromosome 7 abn. : poor – Other abnormalities • Point mutation – RAS, p 53, M-CSFR, NF 1

Clinical manifestation • Anemia • Thrombocytopenia • Leucopenia with qualitative defect • Progress to AML


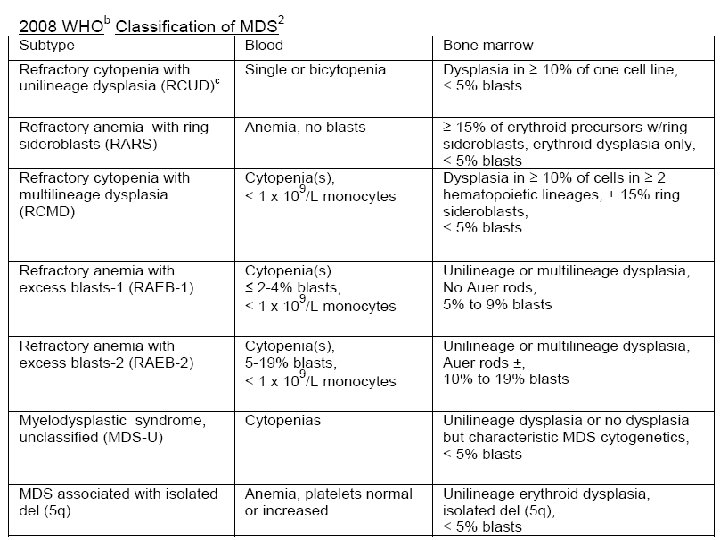
MDS classification (WHO) 1) Refractory cytopenia with unilineage dysplasia 2) 3) 4) 5) 1) Refractory anemia 2) Refractory neutropenia 3) Refractory thrombocytopenia Refractory anemia with ring sideroblasts Refractory cytopenia with multineage dysplasia Refractory anemia with excess blasts ( - II. ) Myelodysplastic syndrome associated with isolated del(5 q) 6) Myelodysplastic syndrome, unclassifiable 7) Childhood myelodysplastic syndrome 1) Refractory cytopenia of childhood

Classification of MDS • FAB § WHO § Refractory cytopenia with – Refractory anemia (RA) unilineage dysplasia (RCUD) – RA with ringed § RC with multilineage dysplasia sideroblasts (RARS) (RCMD) – RA with excess blasts § RA with ringed sideroblasts (RARS) (RAEB) § RA with excess blasts-1 – RAEB in (RAEB-I) transformation § RAEB-II (RAEB-t) § MDS, unclassified (MDS-U) – Chronic § MDS associated with myelomonocytic isolated del(5 q) leukemia (CMML)



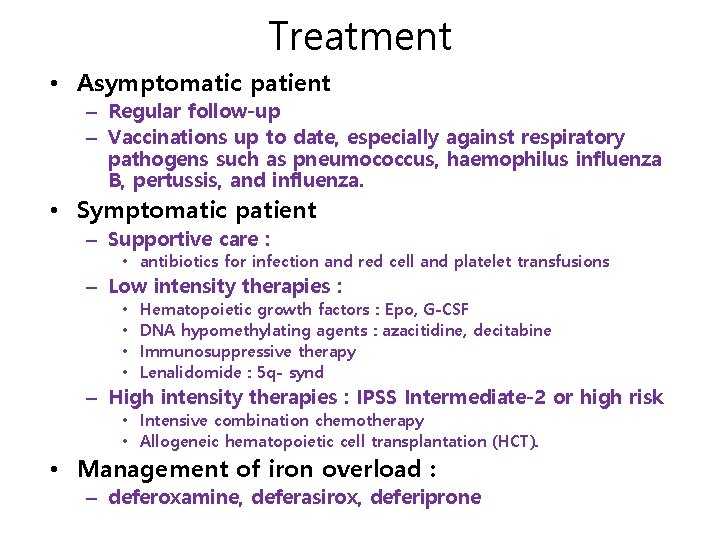
Treatment • Asymptomatic patient – Regular follow-up – Vaccinations up to date, especially against respiratory pathogens such as pneumococcus, haemophilus influenza B, pertussis, and influenza. • Symptomatic patient – Supportive care : • antibiotics for infection and red cell and platelet transfusions – Low intensity therapies : • • Hematopoietic growth factors : Epo, G-CSF DNA hypomethylating agents : azacitidine, decitabine Immunosuppressive therapy Lenalidomide : 5 q- synd – High intensity therapies : IPSS Intermediate-2 or high risk • Intensive combination chemotherapy • Allogeneic hematopoietic cell transplantation (HCT). • Management of iron overload : – deferoxamine, deferasirox, deferiprone

Response criteria for hematologic improvement: • Erythroid response — – Pretreatment Hb <11 g/d. L : increas by ≥ 1. 5 g/d. L for at least 8 weeks – A reduction in the units of red cell transfusions by an absolute number of at least 4 red cell transfusions per 8 weeks compared with the pretreatment transfusion number in the previous 8 weeks. (only red cell transfusions given for a Hb ≤ 9 g/d. L ) • Platelet response — – Pretreatment platelet count <100 x 109/L : • For patients starting with >20 x 109/L : increase of ≥ 30 x 109/L. • For those with from 10 x 109/L to < 20 x 109/L : an increase of at least 100 %. • Neutrophil response — – pretreatment neutrophil count <1 x 109/L : at least 100 percent increase and an absolute >0. 5 x 109/L. increase

Bone marrow response (must persist for a minimum duration of four weeks) • Complete remission (CR) : – ≤ 5 % myeloblasts with normal maturation of all cell lines. – Dysplastic changes may be seen, but within the normal range of dysplastic changes. – Hemoglobin ≥ 11 g/d. L, platelets ≥ 100 x 109/L, neutrophils ≥ 1 x 109/L, and no circulating blasts. • Partial remission (PR) : – • Criteria for CR except BM blasts decreased by ≥ 50 %, but are still >5 % Marrow complete remission : – Marrow complete remission was developed to describe patients who have achieved a complete remission in the bone marrow, but have not recovered peripheral blood counts. • Stable disease : – Failure to achieve at least PR, but no evidence of progression for at least 8 weeks • Disease progression : – Defined by the percent blasts: those with <5 % blasts have a ≥ 50 % increase in blasts to >5 % blasts; those with 5 to 10 % blasts have a ≥ 50 % increase in blasts to >10 %; those with 10 to 20 % blasts have a ≥ 50 % increase to >20 % – Disease progression : an at least 50 % decrement from maximum remission/response in granulocytes or platelets, reduction in Hb by ≥ 2 g/d. L, or transfusion dependence.

Treatment • MDS is a heterogeneous disease with certain subsets of patients surviving for a decade or more with supportive care alone. • With the exception of allogeneic hematopoietic cell transplantation (HCT), MDS cannot be cured by current treatment options. • The main goals of therapy for most patients with MDS are to control symptoms and to improve quality of life, which includes minimizing the toxicity of therapy. • There is no evidence that the treatment of asymptomatic patients improves long term survival.

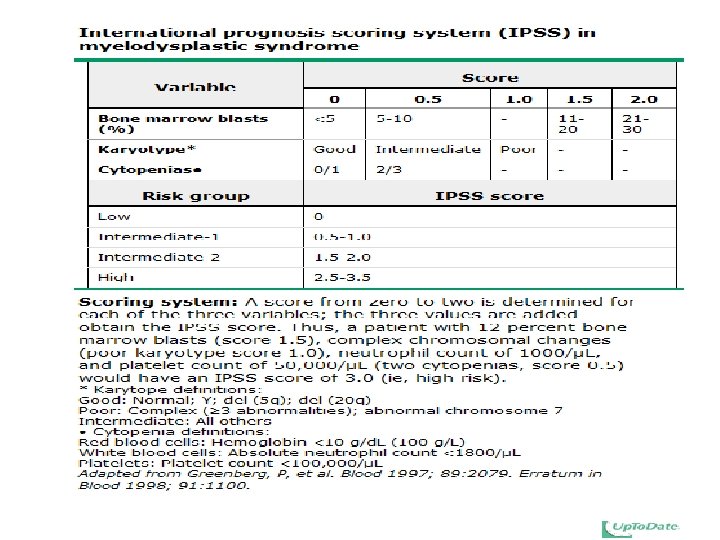
MDS (summary) • Clonal hematologic neoplasms with dysplastic changes (ineffective hematopoiesis) • Classification according to involved lineage, ringed sideroblasts, BM blast count, chromosomal change • Sx : BM failure, transform to acute leukemia • IPSS or WHO prognostic score • Tx: – supportive – chemotherapy – stem cell transplantation

Acute Myeloid Leukemia (AML)

Leukemia Definition: A heterogenous group of neoplasms arising from the hematopoietic cells that proliferate initially in the bone marrow before disseminating to the peripheral blood, spleen, lymph nodes, and ultimately, other tissue

Classification • Cells of origin – Myeloid (non-lymphoid) – Lymphoid • Rapidity of natural history – Acute – Chronic • Morphology and cytogenetics – FAB – WHO

Leukemia • Acute leukemia – Acute myeloid leukemia • M 0 – M 7 FAB – Acute lymphoblastic leukemia : Lymphoblastic lymphoma (NHL) WHO • Chronic leukemia – Chronic myeloid leukemia : MPN – Chronic lymphocytic leukemia : Small lymphocytic lymphoma (NHL) WHO

Acute myeloid leukemia (AML) and related precursor neoplasms

Pathology and classification • Morphologic : – Cytochemistry; FAB classification • Immunophenotypic : – Surface markers (CDs) • Chromosomal: – Translocations etc • Molecular

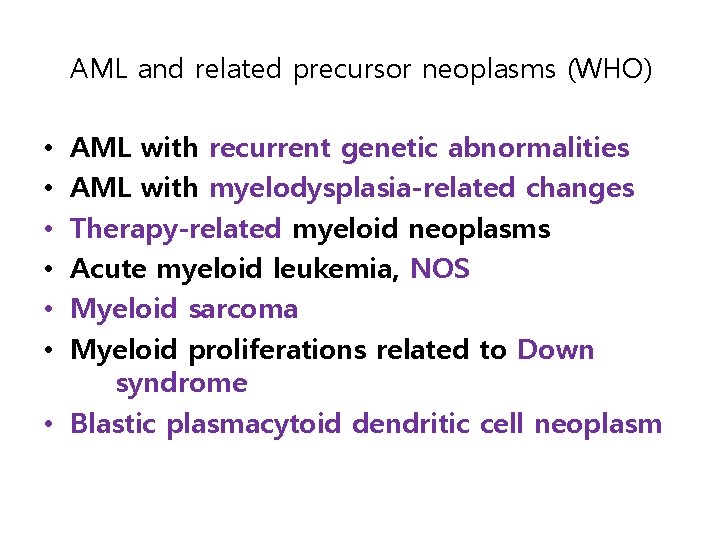
AML and related precursor neoplasms (WHO) AML with recurrent genetic abnormalities AML with myelodysplasia-related changes Therapy-related myeloid neoplasms Acute myeloid leukemia, NOS Myeloid sarcoma Myeloid proliferations related to Down syndrome • Blastic plasmacytoid dendritic cell neoplasm • • •

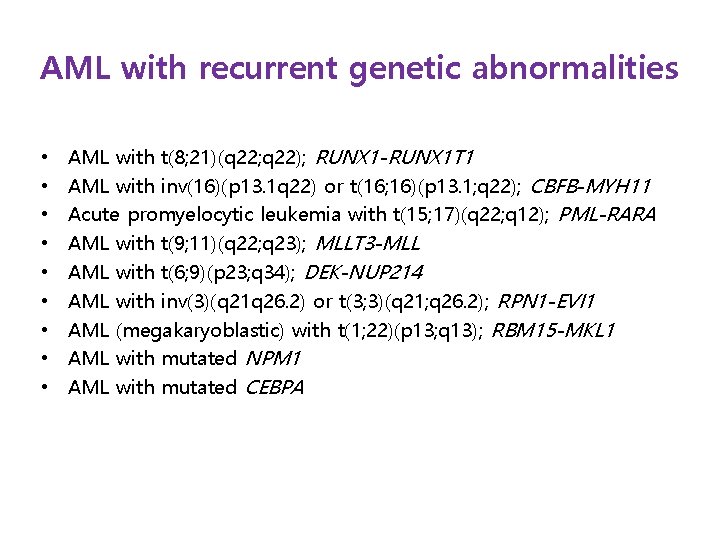
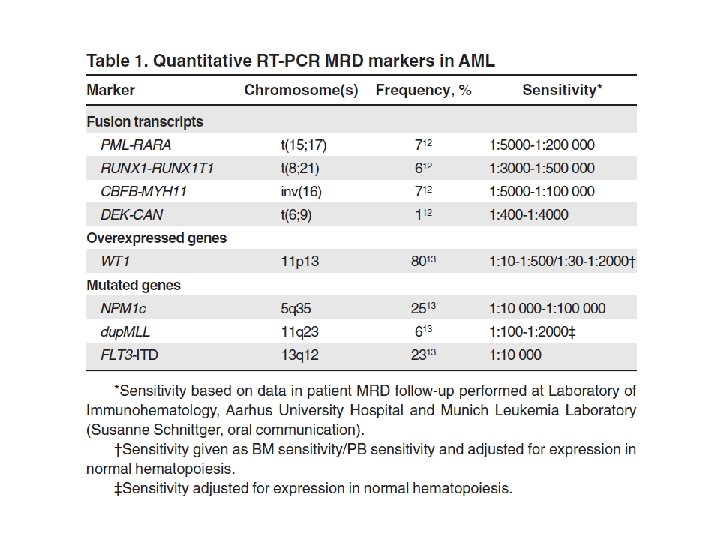
AML with recurrent genetic abnormalities • • • AML with t(8; 21)(q 22; q 22); RUNX 1 -RUNX 1 T 1 AML with inv(16)(p 13. 1 q 22) or t(16; 16)(p 13. 1; q 22); CBFB-MYH 11 Acute promyelocytic leukemia with t(15; 17)(q 22; q 12); PML-RARA AML with t(9; 11)(q 22; q 23); MLLT 3 -MLL AML with t(6; 9)(p 23; q 34); DEK-NUP 214 AML with inv(3)(q 21 q 26. 2) or t(3; 3)(q 21; q 26. 2); RPN 1 -EVI 1 AML (megakaryoblastic) with t(1; 22)(p 13; q 13); RBM 15 -MKL 1 AML with mutated NPM 1 AML with mutated CEBPA

Acute myeloid leukemia, NOS • • • AML with minimal differentiation AML without maturation AML with maturation Acute myelomonocytic leukemia Acute monoblastic and monocytic leukemia Acute erythroid leukemia Acute megakaryoblastic leukemia Acute basophilic leukemia Acute panmyelosis with myelofibrosis (Myeloid sarcoma)


AML • Incidence: 2. 3 per 100, 000 per year • Etiology: – Hereditary: Down, Klinefelter, Patau, Fanconi, Bloom, ataxia telangiectasia, Kostman – Radiation – Chemical: benzene – Drug: alkylating agents, epipodophyllotoxins


Clinical presentation • Symptoms: – – – Neutropenia: infection Leukocytosis: leucostasis Thrombocytopenia: bleeding; M 3 --DIC Anemia bone pain, headache, diaphoresis, cough • Physical findings – fever, splenomegaly, hepatomegaly, lymphadenopathy, sternal tenderness – Gingival, skin, soft tissue or meningeal infiltration (M 4, M 5)

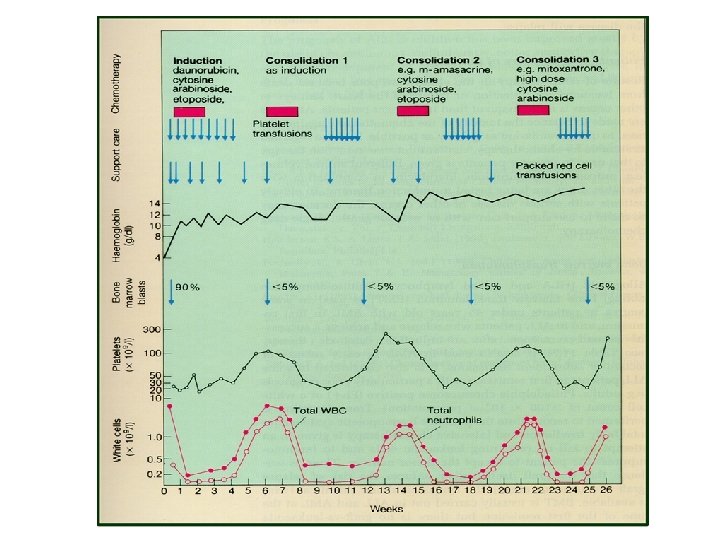
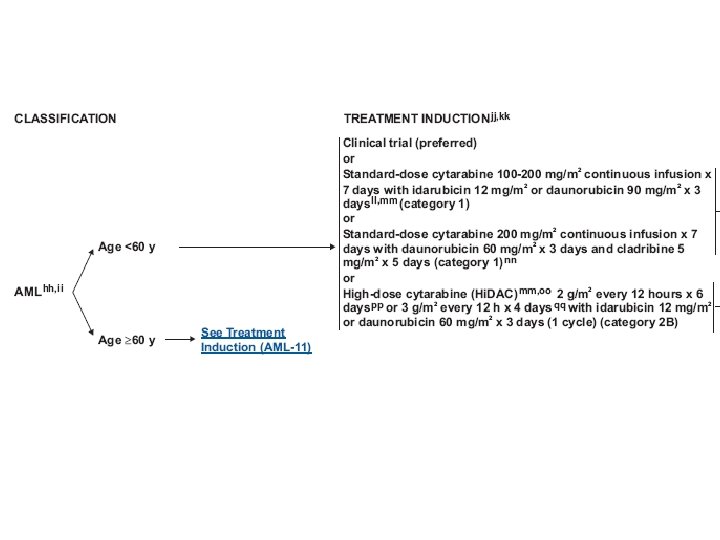
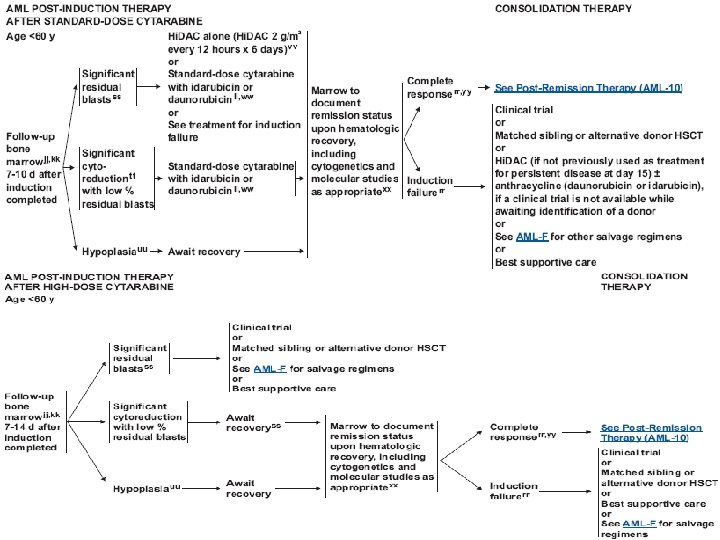
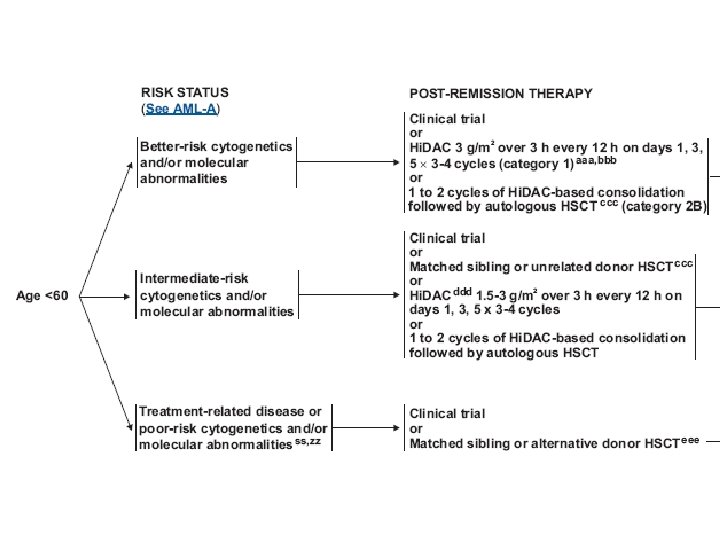
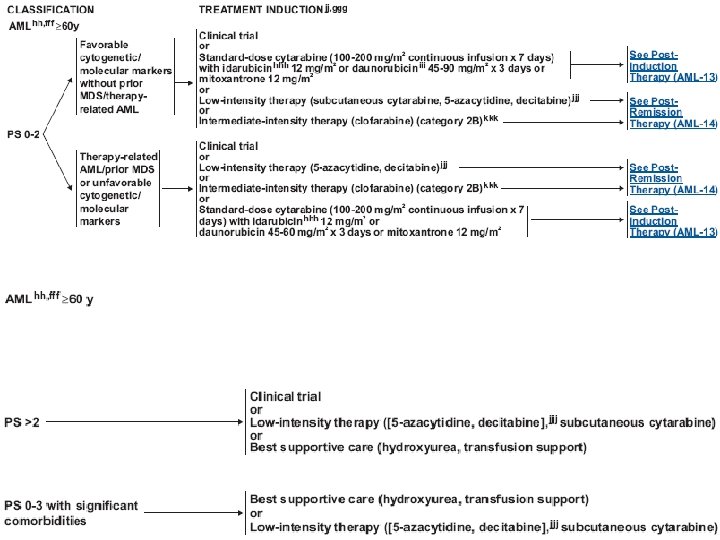
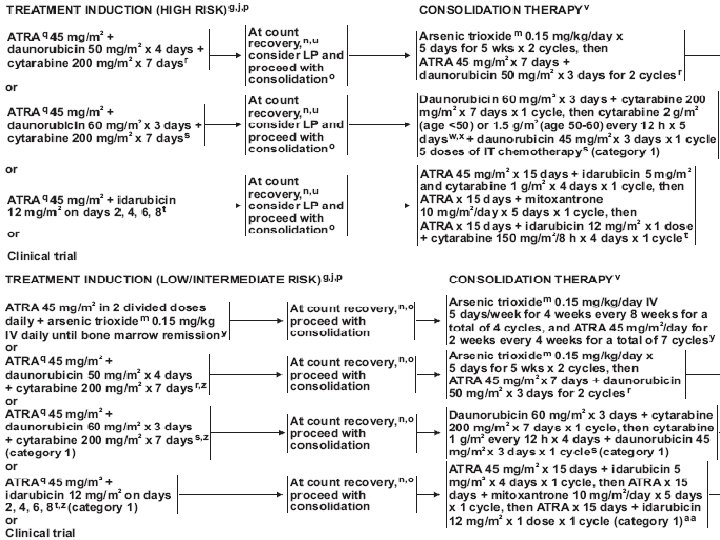
Treatment (AML) • Induction chemotherapy • Postremission therapy – Consolidation – Hematopoietic cell transplantation • Supportive care – Blood transfusion – Infection control – Hematopoietic growth factors


Treatment (AML) • Induction : anthracyclin (daunorubicin, idarubicin), cytarabine, cladribine • Consolidation : same as induction, or high dose cytarabine • Others : etoposide, mitoxantrone, fludarabine, clofarabine • Hypomethylating agents : 5 -azacytidine, decitabine





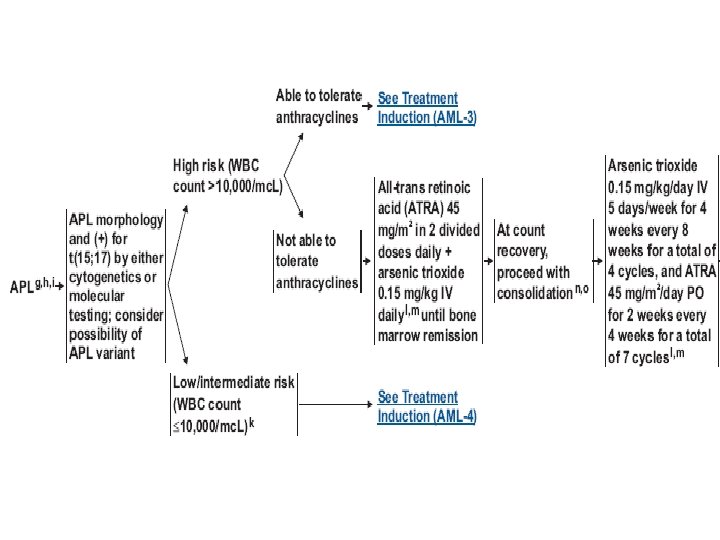
APL(M 3) • • leukopenia DIC t(15; 17): PML-RARA fusion gene ATRA (all-trans retinoic acid) – retinoic acid syndrome • Arsenic trioxide • Chemotherapy • Good prognosis





20 Blast 5 CR

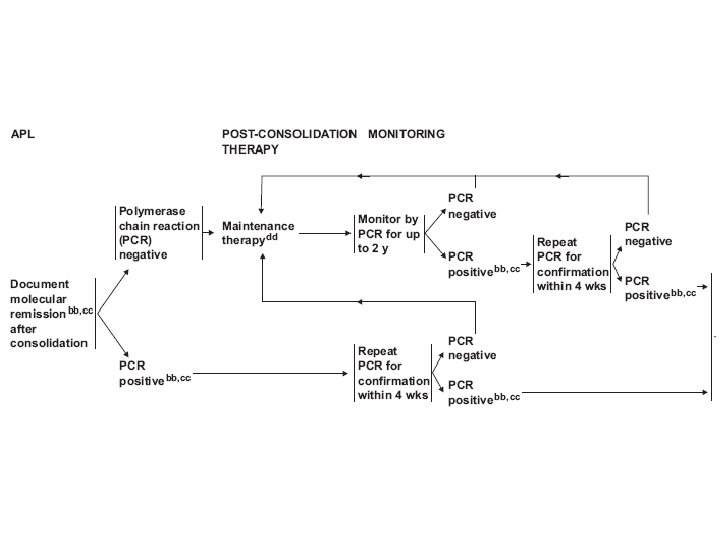
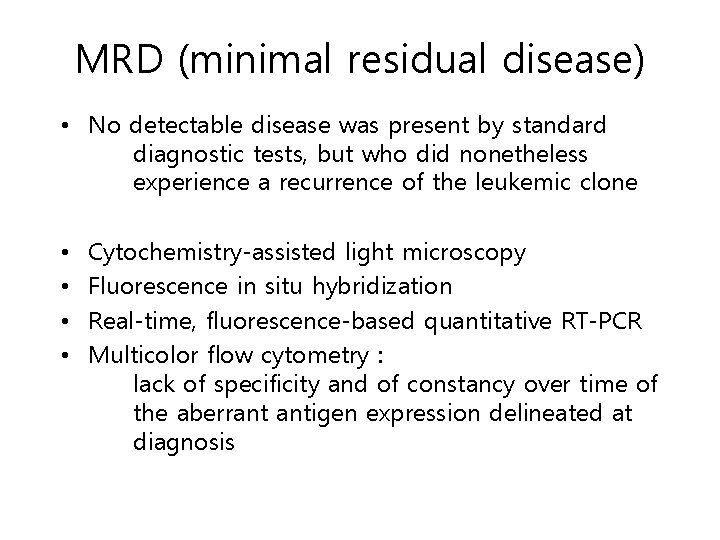
MRD (minimal residual disease) • No detectable disease was present by standard diagnostic tests, but who did nonetheless experience a recurrence of the leukemic clone • • Cytochemistry-assisted light microscopy Fluorescence in situ hybridization Real-time, fluorescence-based quantitative RT-PCR Multicolor flow cytometry : lack of specificity and of constancy over time of the aberrant antigen expression delineated at diagnosis


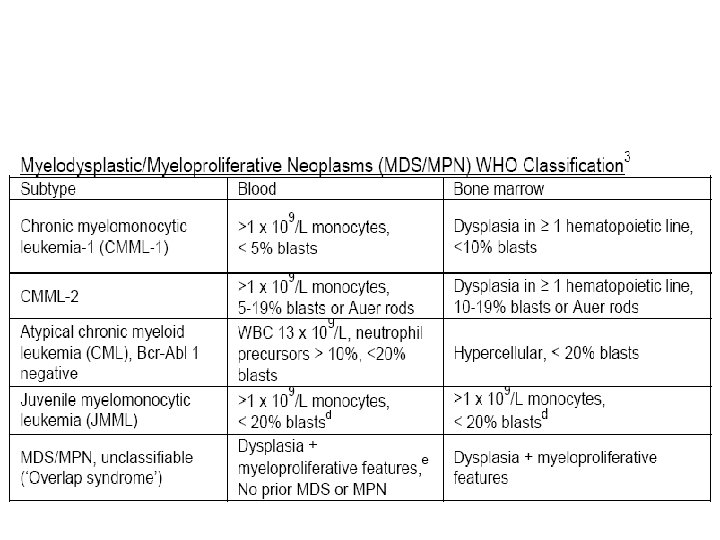
Myelodysplastic/Myeloproliferative Neoplasm (MDS/MPN)

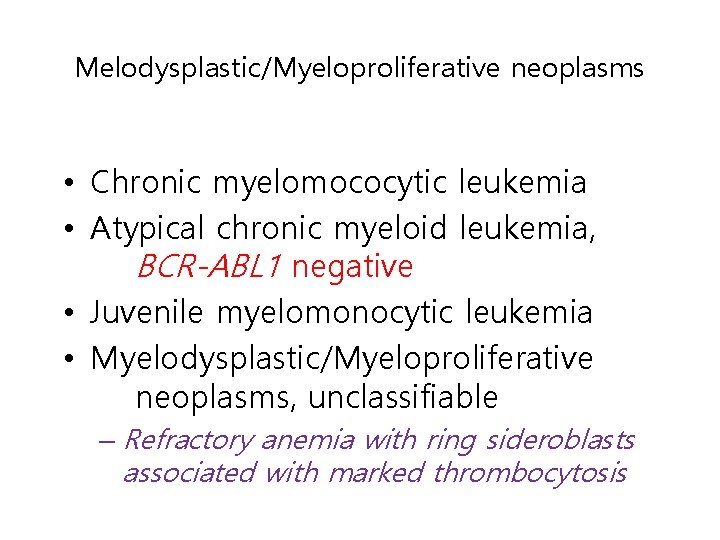
Melodysplastic/Myeloproliferative neoplasms • Chronic myelomococytic leukemia • Atypical chronic myeloid leukemia, BCR-ABL 1 negative • Juvenile myelomonocytic leukemia • Myelodysplastic/Myeloproliferative neoplasms, unclassifiable – Refractory anemia with ring sideroblasts associated with marked thrombocytosis


Treatment of MDS/MPN • Symptomatic • Chemotherapy – As MDS – As AML • Allogeneic SCT