2009 PrenticeHall Inc HAVE YOU EVER WONDERED Why












- Slides: 42

2009, Prentice-Hall, Inc.


HAVE YOU EVER WONDERED § Why the stars shine? § Why ice melts and water evaporates? § Why do leaves turn colors in the fall, and how does a battery generate electricity? § Why does keeping foods cold slow their spoilage, and how do our bodies use food to maintain life?

CHEMISTRY IS… § Chemistry is the study of matters and the changes it undergoes. § How do chemical principles operate in all aspects of our lives?

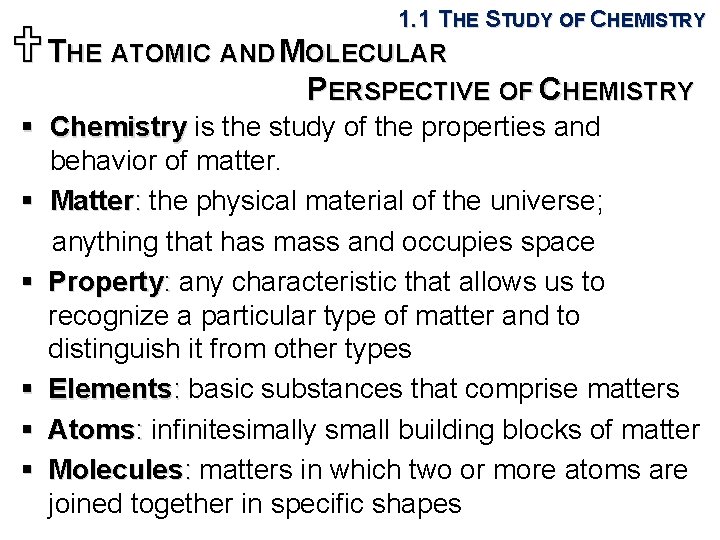
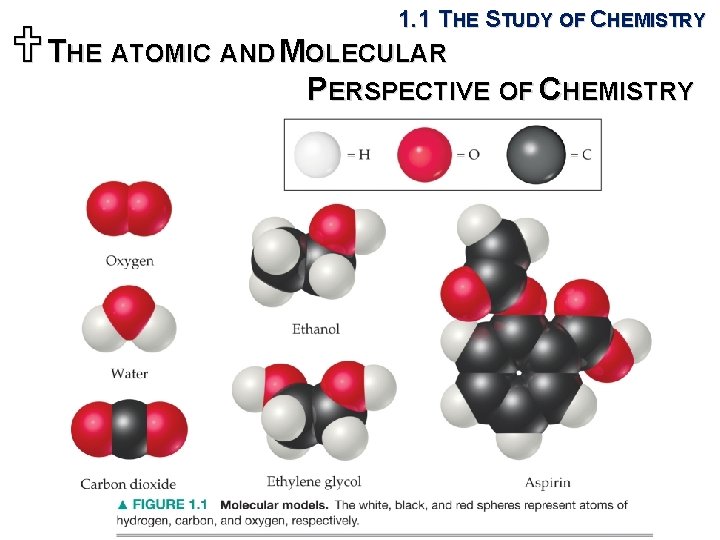
1. 1 THE STUDY OF CHEMISTRY UTHE ATOMIC AND MOLECULAR PERSPECTIVE OF CHEMISTRY § Chemistry is the study of the properties and behavior of matter. § Matter: the physical material of the universe; anything that has mass and occupies space § Property: any characteristic that allows us to recognize a particular type of matter and to distinguish it from other types § Elements: basic substances that comprise matters § Atoms: infinitesimally small building blocks of matter § Molecules: matters in which two or more atoms are joined together in specific shapes

1. 1 THE STUDY OF CHEMISTRY UTHE ATOMIC AND MOLECULAR PERSPECTIVE OF CHEMISTRY

1. 1 THE STUDY OF CHEMISTRY UWHY STUDY CHEMISTRY? § To satisfy our curiosity? § Because it is an essential part of your curriculum? § To understand our world and how it works FIGURE 1. 2 Chemistry helps us understand the world around us.

1. 1 THE STUDY OF CHEMISTRY UWHY STUDY CHEMISTRY? § To improve health care § To provide our everyday needs for food, clothing, and shelter § Chemistry helps us better understand materials § The central science

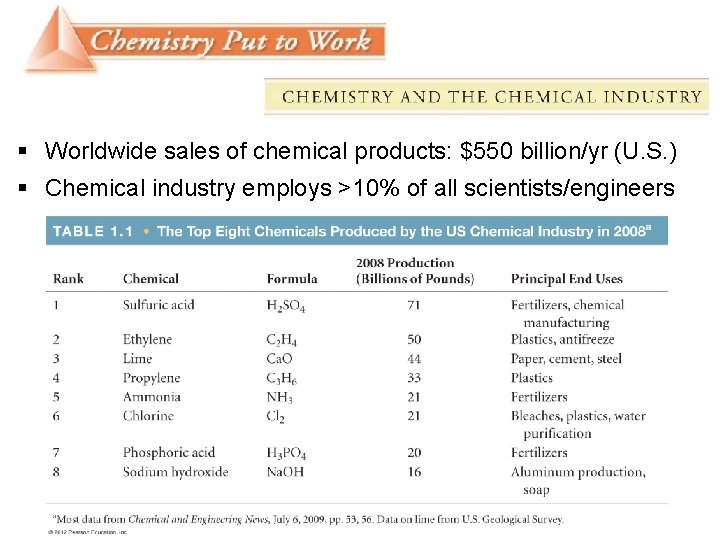
§ Worldwide sales of chemical products: $550 billion/yr (U. S. ) § Chemical industry employs >10% of all scientists/engineers


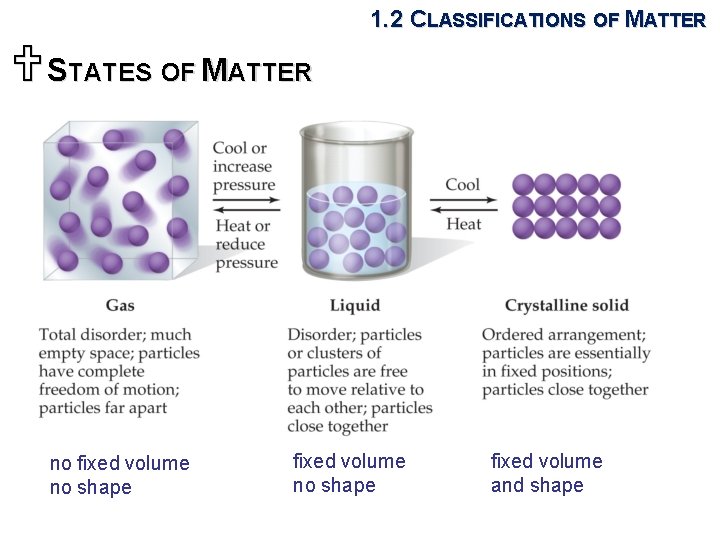
1. 2 CLASSIFICATIONS OF MATTER USTATES OF MATTER no fixed volume no shape fixed volume and shape

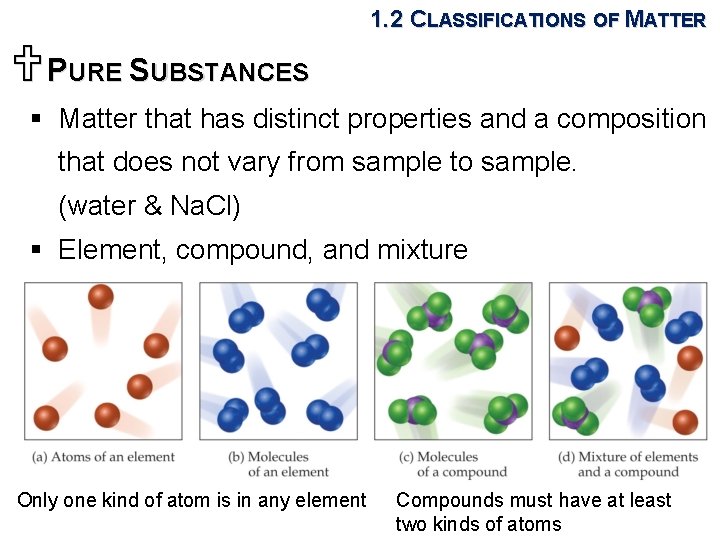
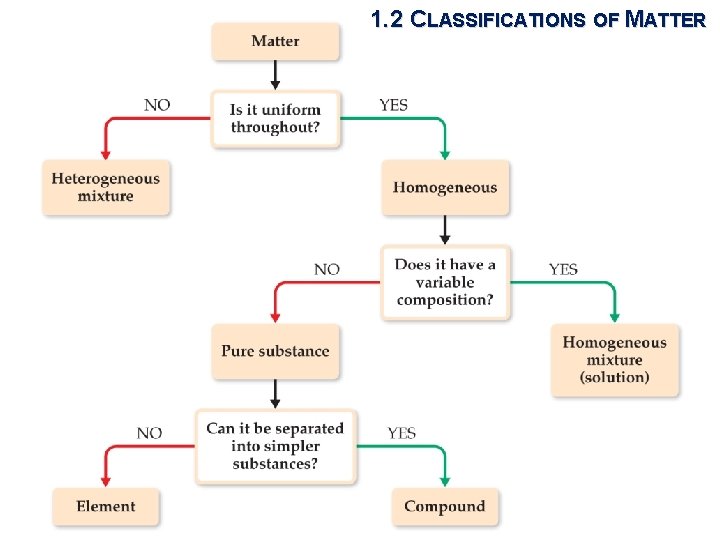
1. 2 CLASSIFICATIONS OF MATTER UPURE SUBSTANCES § Matter that has distinct properties and a composition that does not vary from sample to sample. (water & Na. Cl) § Element, compound, and mixture Only one kind of atom is in any element Compounds must have at least two kinds of atoms

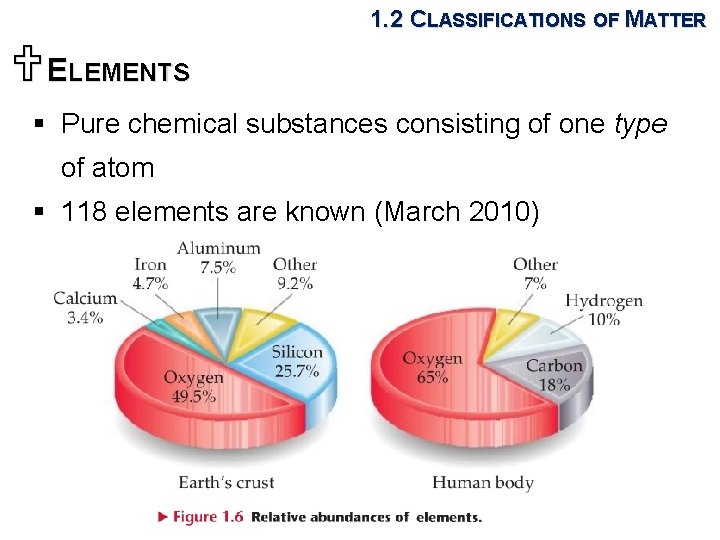
1. 2 CLASSIFICATIONS OF MATTER UELEMENTS § Pure chemical substances consisting of one type of atom § 118 elements are known (March 2010)

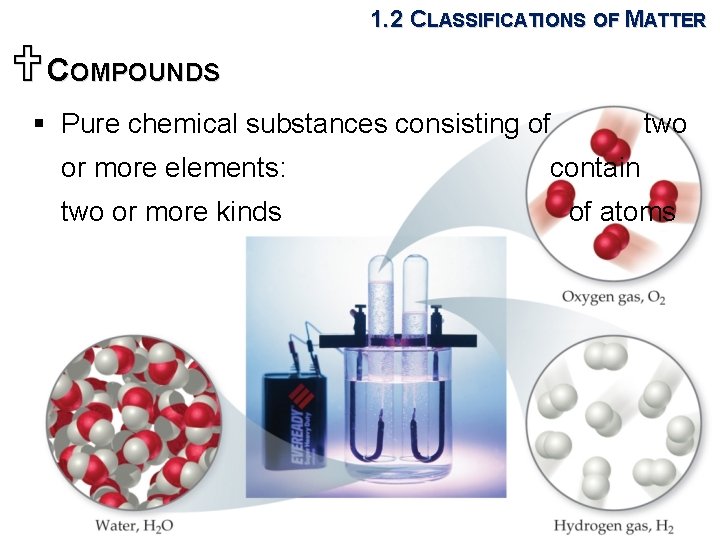
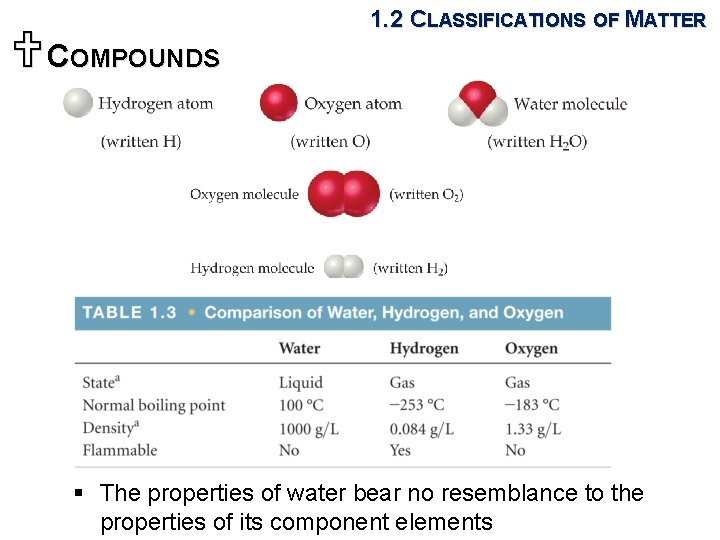
1. 2 CLASSIFICATIONS OF MATTER UCOMPOUNDS § Pure chemical substances consisting of or more elements: two or more kinds two contain of atoms

UCOMPOUNDS 1. 2 CLASSIFICATIONS OF MATTER § The properties of water bear no resemblance to the properties of its component elements

1. 2 CLASSIFICATIONS OF MATTER UMIXTURES § Heterogeneous mixtures do not have the same composition, properties, and appearance throughout § Homogeneous mixtures have the same composition, properties, and appearance throughout (solutions) Rock Copper sulfate

1. 2 CLASSIFICATIONS OF MATTER

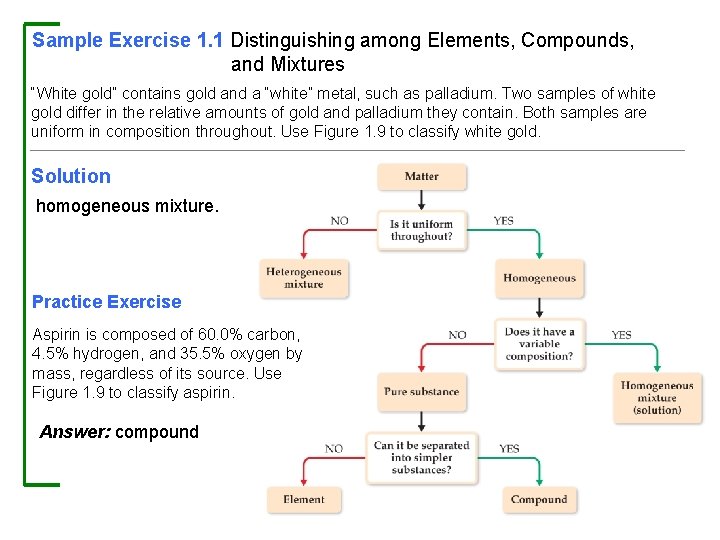
Sample Exercise 1. 1 Distinguishing among Elements, Compounds, and Mixtures “White gold” contains gold and a “white” metal, such as palladium. Two samples of white gold differ in the relative amounts of gold and palladium they contain. Both samples are uniform in composition throughout. Use Figure 1. 9 to classify white gold. Solution homogeneous mixture. Practice Exercise Aspirin is composed of 60. 0% carbon, 4. 5% hydrogen, and 35. 5% oxygen by mass, regardless of its source. Use Figure 1. 9 to classify aspirin. Answer: compound

1. 3 PROPERTIES OF MATTER UTYPES OF PROPERTIES § Physical Properties… • Can be observed without changing a substance into another substance. • Boiling point, density, mass, volume, etc. § Chemical Properties… • Can only be observed when a substance is changed into another substance. • Flammability, corrosiveness, reactivity with acid, etc.

1. 3 PROPERTIES OF MATTER UTYPES OF PROPERTIES § Intensive Properties… • Are independent of the amount of the substance that is present. • Temperature, density, boiling point, color, etc. § Extensive Properties… • Depend upon the amount of the substance present. • Mass, volume, etc.

1. 3 PROPERTIES OF MATTER UTYPES OF PROPERTIES § Physical Changes • These are changes in matter that do not change the composition of a substance. • Changes of state, temperature, volume, etc. § Chemical Changes • Chemical changes result in new substances. • Combustion, oxidation, decomposition, etc.

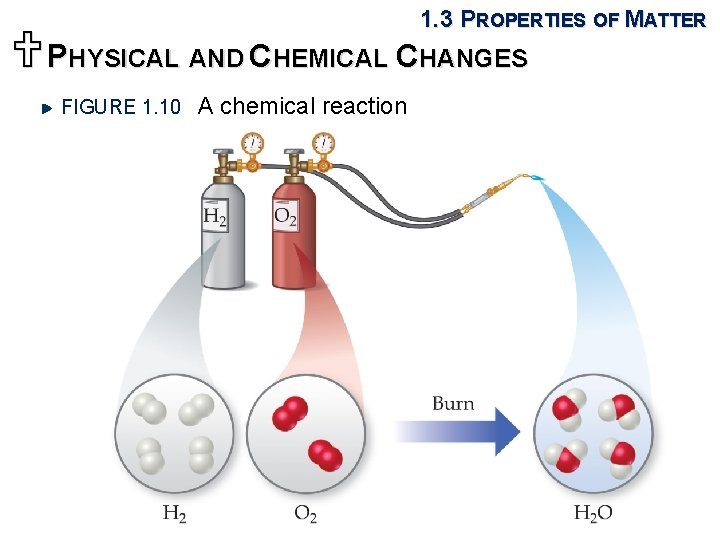
1. 3 PROPERTIES OF MATTER UPHYSICAL AND CHEMICAL CHANGES FIGURE 1. 10 A chemical reaction

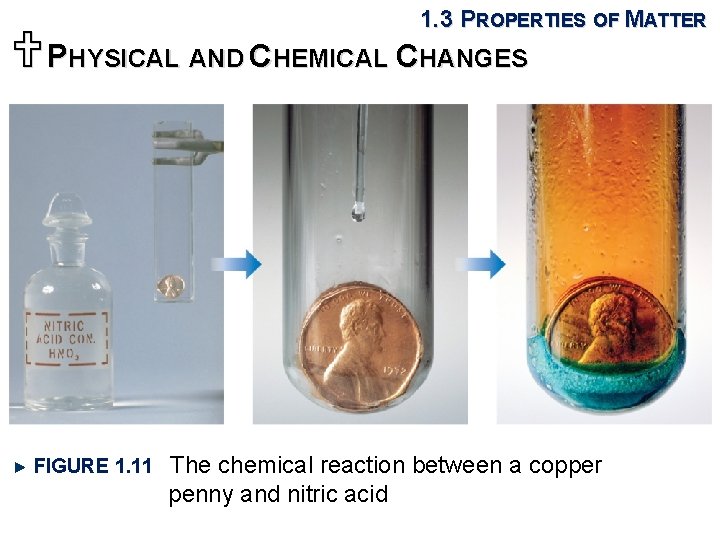
1. 3 PROPERTIES OF MATTER UPHYSICAL AND CHEMICAL CHANGES FIGURE 1. 11 The chemical reaction between a copper penny and nitric acid

USEPARATION OF MIXTURES 1. 3 PROPERTIES OF MATTER § In filtration, solid substances are separated from liquids and solutions. § Salt / copper FIGURE 1. 12 Separation by filtration

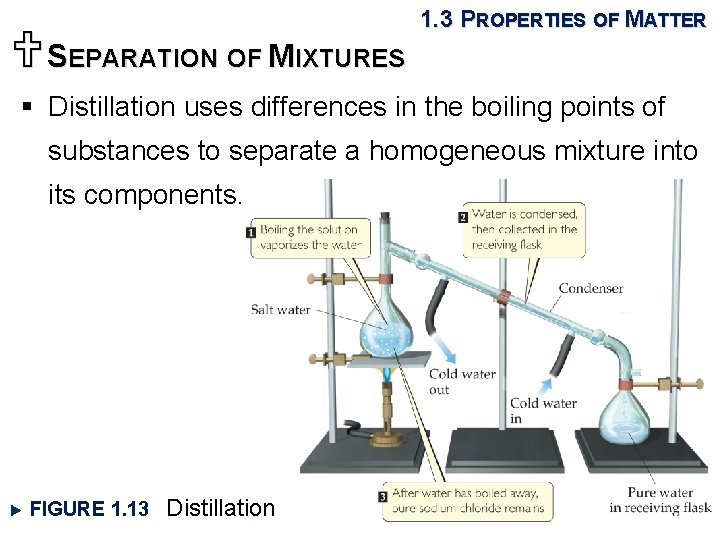
USEPARATION OF MIXTURES 1. 3 PROPERTIES OF MATTER § Distillation uses differences in the boiling points of substances to separate a homogeneous mixture into its components. FIGURE 1. 13 Distillation

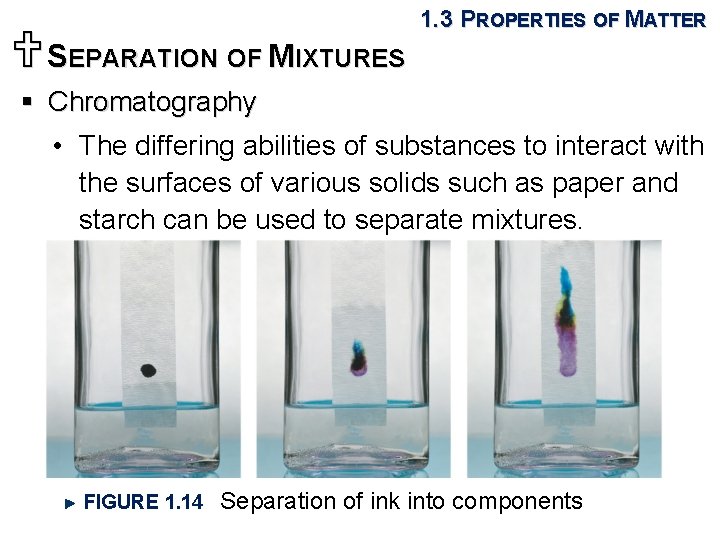
USEPARATION OF MIXTURES 1. 3 PROPERTIES OF MATTER § Chromatography • The differing abilities of substances to interact with the surfaces of various solids such as paper and starch can be used to separate mixtures. FIGURE 1. 14 Separation of ink into components

1. 4 UNITS OF MEASUREMENT UTHE METRIC SYSTEM AND SI UNITS § The metric system • An international system of measurement, first adopted by France in 1791, that is the common system of measuring units used by most of the world. FIGURE 1. 15 Metric units. Fluid ounces (fl oz), English units; m. L, metric units

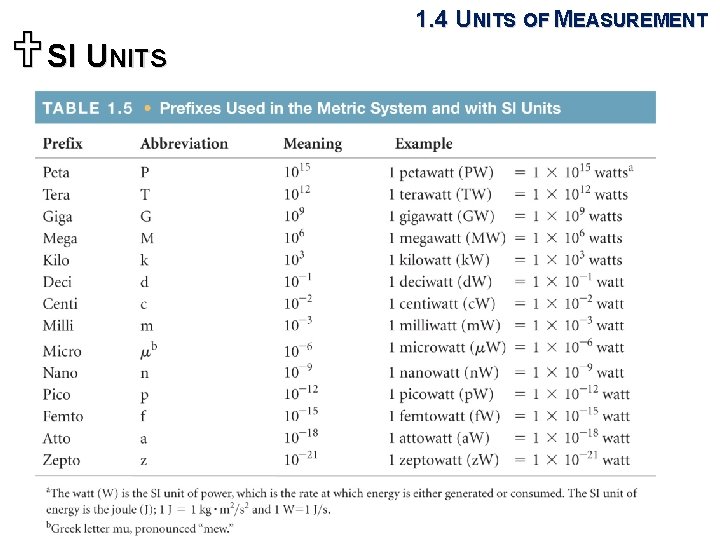
USI UNITS 1. 4 UNITS OF MEASUREMENT § The world's most widely used system of measurement, both in everyday commerce and in science. § From seven base units, all other units are derived

USI UNITS 1. 4 UNITS OF MEASUREMENT

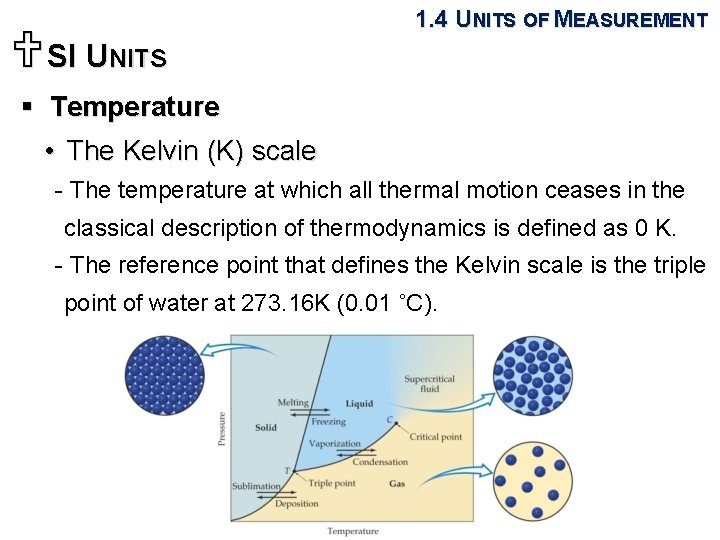
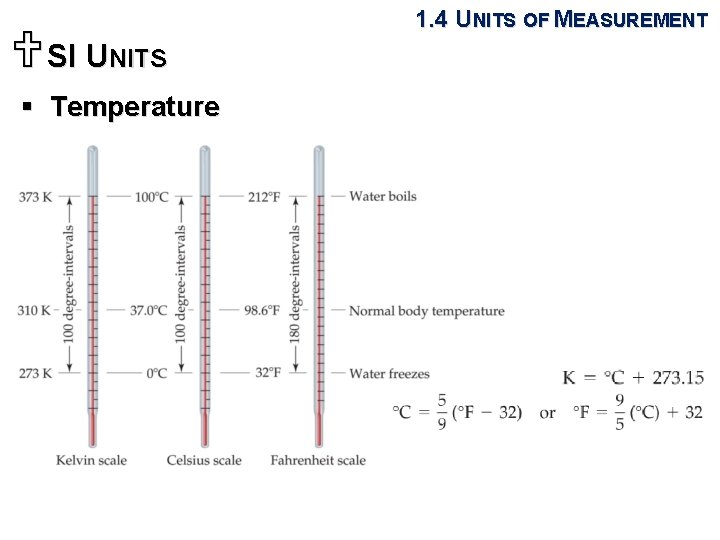
USI UNITS 1. 4 UNITS OF MEASUREMENT § Length and mass • The SI base units of length and mass are the meter (m) and kilogram (kg). § Temperature • A measure of the hotness or coldness of an object. • The Celsius (˚C) scale: the freezing point (0 ˚C) and boiling point (100 ˚C) of water. • The Kelvin (K) scale: The SI unit of temperature.

USI UNITS 1. 4 UNITS OF MEASUREMENT § Temperature • The Kelvin (K) scale - The temperature at which all thermal motion ceases in the classical description of thermodynamics is defined as 0 K. - The reference point that defines the Kelvin scale is the triple point of water at 273. 16 K (0. 01 ˚C).

USI UNITS § Temperature 1. 4 UNITS OF MEASUREMENT

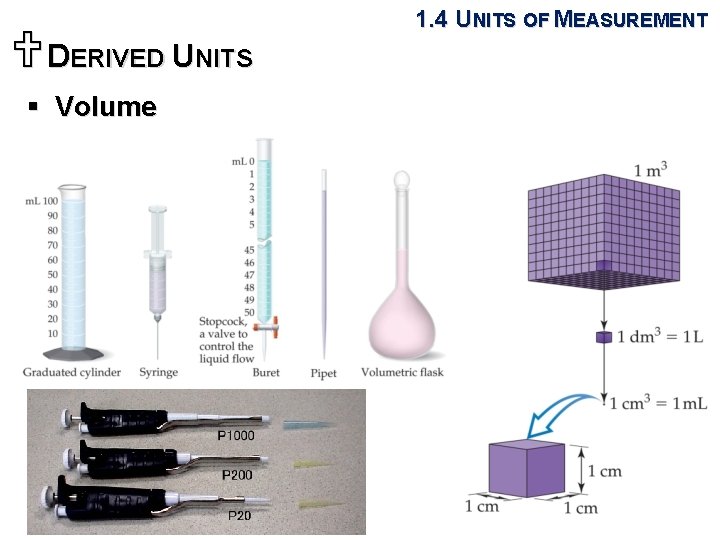
UDERIVED UNITS § Volume 1. 4 UNITS OF MEASUREMENT

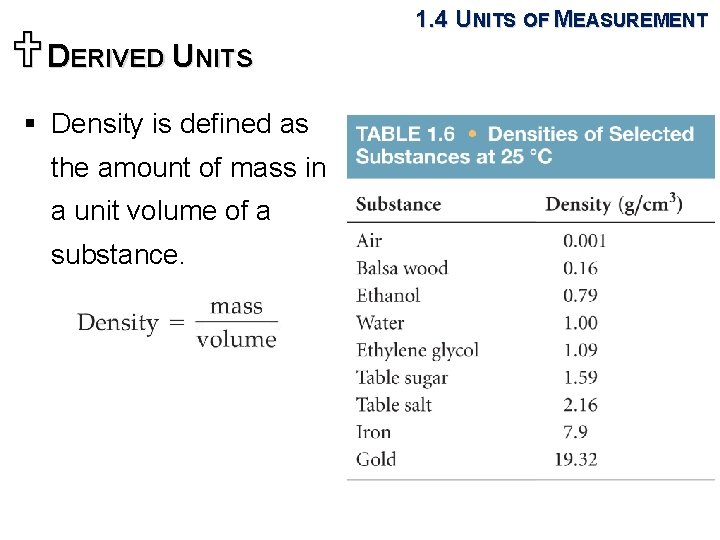
UDERIVED UNITS 1. 4 UNITS OF MEASUREMENT § Density is defined as the amount of mass in a unit volume of a substance. ( g/cm or g/m. L)

1. 5 UNCERTAINTY IN MEASUREMENT UEXACT NUMBERS AND INEXACT NUMBERS § Exact numbers • 12 eggs in a dozen • 1000 g in a kilogram • 1 kg = 2. 2046 lb § Inexact numbers • Numbers obtained by measurement • Uncertainties always exist in measured quantities.

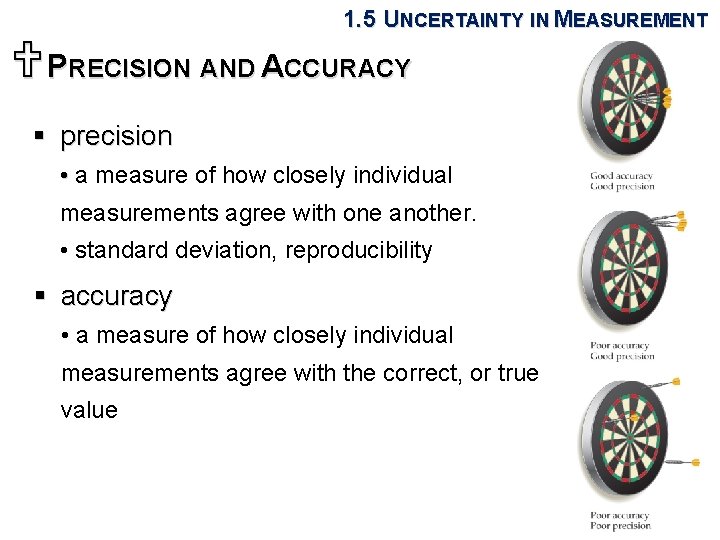
1. 5 UNCERTAINTY IN MEASUREMENT UPRECISION AND ACCURACY § precision • a measure of how closely individual measurements agree with one another. • standard deviation, reproducibility § accuracy • a measure of how closely individual measurements agree with the correct, or true value

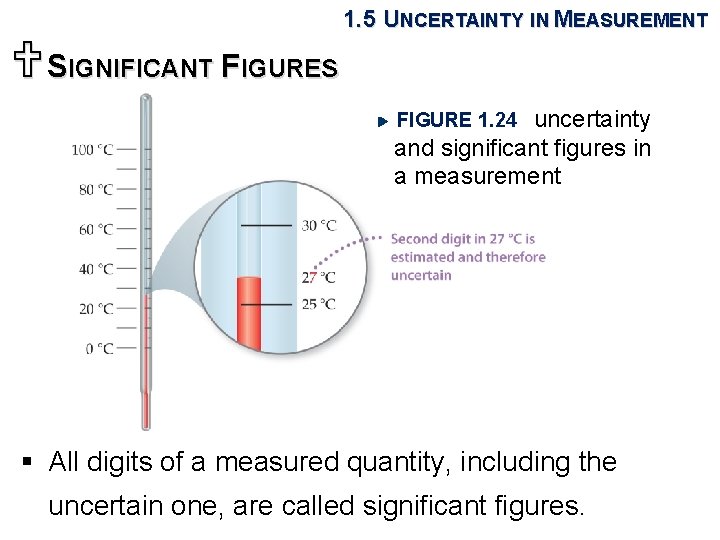
1. 5 UNCERTAINTY IN MEASUREMENT USIGNIFICANT FIGURES uncertainty and significant figures in a measurement FIGURE 1. 24 § All digits of a measured quantity, including the uncertain one, are called significant figures.

1. 5 UNCERTAINTY IN MEASUREMENT USIGNIFICANT FIGURES

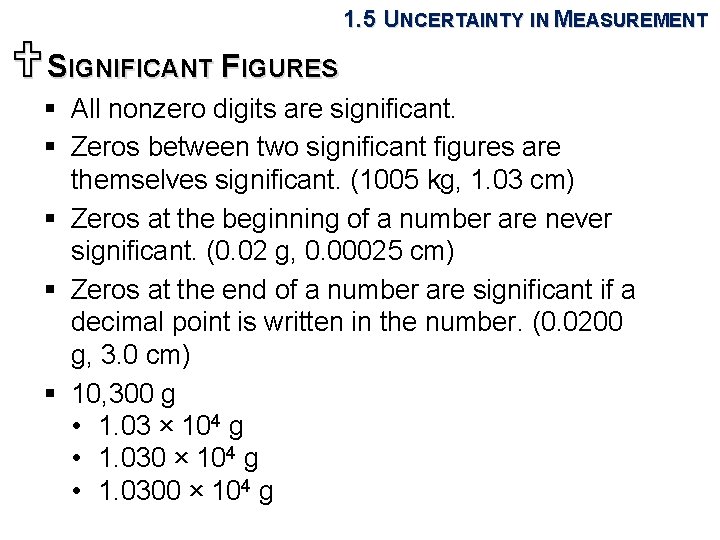
1. 5 UNCERTAINTY IN MEASUREMENT USIGNIFICANT FIGURES § All nonzero digits are significant. § Zeros between two significant figures are themselves significant. (1005 kg, 1. 03 cm) § Zeros at the beginning of a number are never significant. (0. 02 g, 0. 00025 cm) § Zeros at the end of a number are significant if a decimal point is written in the number. (0. 0200 g, 3. 0 cm) § 10, 300 g • 1. 03 × 104 g • 1. 0300 × 104 g

1. 5 UNCERTAINTY IN MEASUREMENT USIGNIFICANT FIGURES

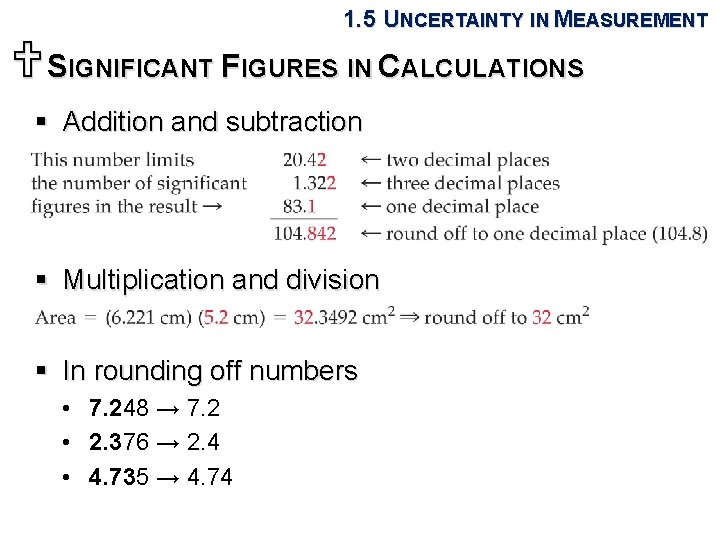
1. 5 UNCERTAINTY IN MEASUREMENT USIGNIFICANT FIGURES IN CALCULATIONS § Addition and subtraction § Multiplication and division § In rounding off numbers • 7. 248 → 7. 2 • 2. 376 → 2. 4 • 4. 735 → 4. 74

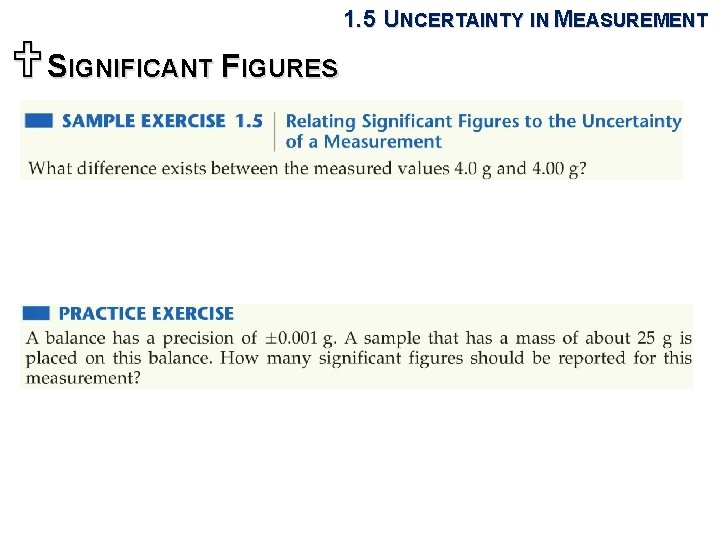
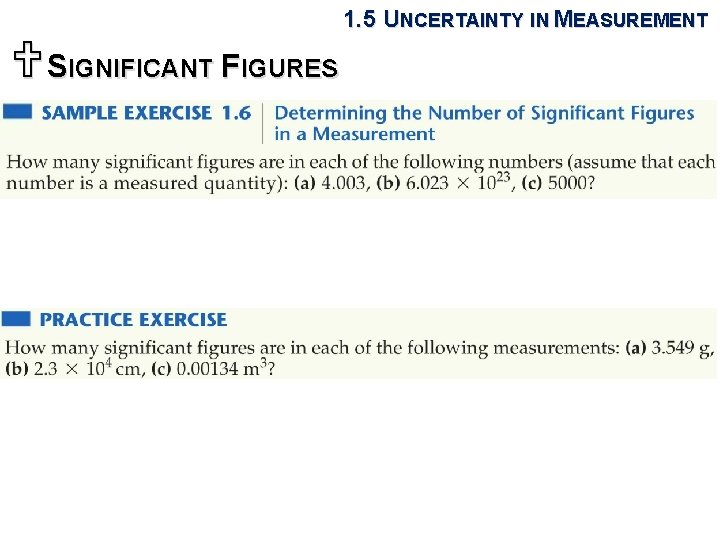
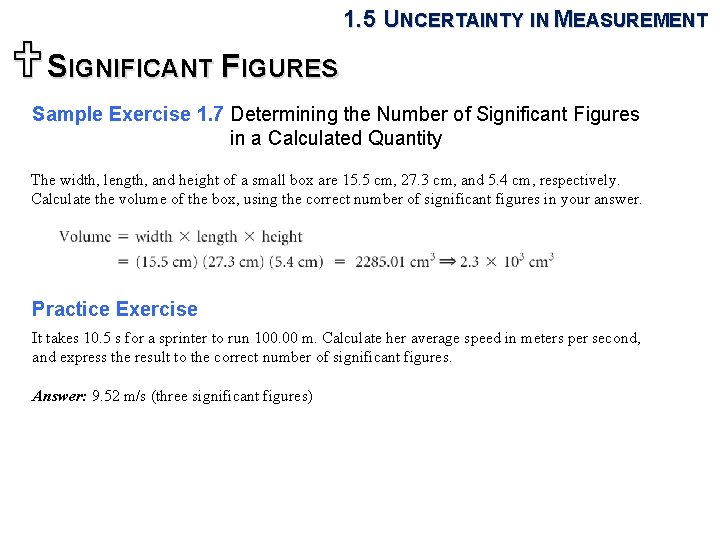
1. 5 UNCERTAINTY IN MEASUREMENT USIGNIFICANT FIGURES Sample Exercise 1. 7 Determining the Number of Significant Figures in a Calculated Quantity The width, length, and height of a small box are 15. 5 cm, 27. 3 cm, and 5. 4 cm, respectively. Calculate the volume of the box, using the correct number of significant figures in your answer. Practice Exercise It takes 10. 5 s for a sprinter to run 100. 00 m. Calculate her average speed in meters per second, and express the result to the correct number of significant figures. Answer: 9. 52 m/s (three significant figures)