2003118 Nation Cheng Kung Univ NCKUPRL Polym Res
























- Slides: 77

奈米化 與高分子材料 • 2003/11/8 • 報告人: 吳逸謨 Nation Cheng Kung Univ. NCKU-PRL Polym. Res. Lab.



奈米福碳公司 -2003奈米科技論壇 Dr. Alan Mac. Dirmid – (2002 Chemistry Nobel-Prize Laureate for conducting polymers) He said Taiwan could someday become a 奈米島 Nation Cheng Kung Univ. NCKU-PRL Polym. Res. Lab.

Analyses of Crystal Forms in Syndiotactic Polystyrene Intercalated with Layered Nano. Clays Nation Cheng Kung Univ. NCKU-PRL Polym. Res. Lab.

高分子 – 超奈米 Nation Cheng Kung Univ. NCKU-PRL Polym. Res. Lab.

Introduction Over the last decade, polymer-layered silicate nanocomposites (PLSNs) with nano-scale reinforcements have been the focus of extensive study. Incorporation of the organo-layered silicates into the polymers results in enhanced mechanical strength, improved thermal stability and flame retardancy, increased solvent resistance and barrier properties, higher ionic conductivity, reduced thermal expansion coefficient, and controlled biodegradability. Dispersivity of the clay particles plays the pivotal role to influence the ultimate properties of the PLSNs. For a typical polymer, the key factors of the clay dispersion are the method of synthesis, structure of the intercalant (generally onium NCKU-PRL surfactants) and concentration of clay. Nation Cheng Kung Univ. Polym. Res. Lab.

Objectives s. PS is a semi-crystalline polymer exhibiting complex polymorphic behavior with multiple melting characteristics. The main objectives of the present investigation are: (1) to elucidate the multiple melting characteristics of s. PS/clay nanocomposites vs. conventional micro-composites by comprehensive thermal and X-ray diffraction evidence; (2) to investigate the effect of Tc on the relative ratio of the polymorphic s. PS crystals in presence of pristine and organoclays; and (3) to depict the spherulitic morphology of s. PS as affected by incorporation of different kind of clays. Nation Cheng Kung Univ. NCKU-PRL Polym. Res. Lab.

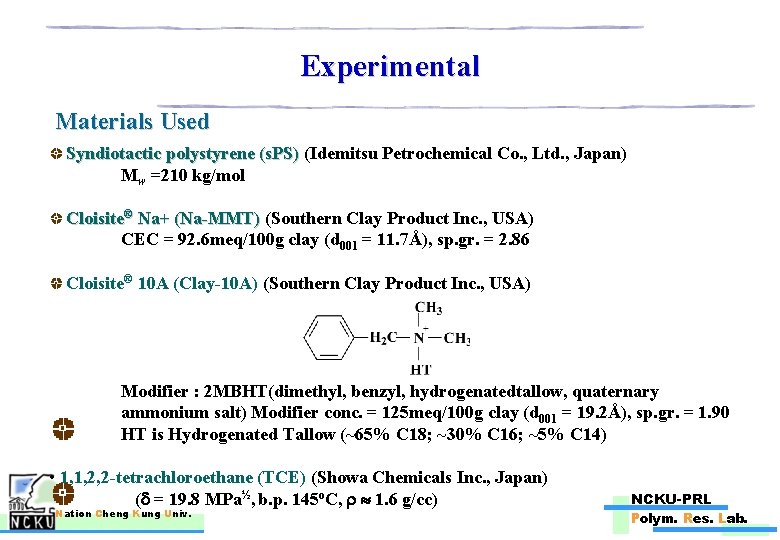
Experimental Materials Used Syndiotactic polystyrene (s. PS) (Idemitsu Petrochemical Co. , Ltd. , Japan) Mw =210 kg/mol Cloisite® Na+ (Na-MMT) (Southern Clay Product Inc. , USA) Na+ (Na-MMT) CEC = 92. 6 meq/100 g clay (d 001 = 11. 7Å), sp. gr. = 2. 86 Cloisite® 10 A (Clay-10 A) (Southern Clay Product Inc. , USA) Modifier : 2 MBHT(dimethyl, benzyl, hydrogenatedtallow, quaternary ammonium salt) Modifier conc. = 125 meq/100 g clay (d 001 = 19. 2Å), sp. gr. = 1. 90 HT is Hydrogenated Tallow (~65% C 18; ~30% C 16; ~5% C 14) • 1, 1, 2, 2 -tetrachloroethane (TCE) (Showa Chemicals Inc. , Japan) ( = 19. 8 MPa½, b. p. 145 o. C, 1. 6 g/cc) Nation Cheng Kung Univ. NCKU-PRL Polym. Res. Lab.

Experimental (contd. ) Instrumentation Differential Scanning Calorimetry (DSC): DSC-7 (Perkin-Elmer) attached with cooling accessory Intracooler 2 (Perkin-Elmer). Wide-Angle X-ray Diffraction (WAXD): Shimadzu XRD-6000 with Cu Kα radiation (30 k. V and 40 m. A) and a wavelength of 1. 542 Å. The scanning 2θ angle ranged between 2° and 30° with a step scanning rate of 2°/min. • Transmission Electron Microscopy (TEM): JEOL JEM 1200 -EX TEM. Epoxy-mounted samples were ultra-microtomed with a diamond knife on a Leica Ultracut R microtme (nominal thickness of 50~70 nm). The sections were transferred from water to carbon coated 200 -mesh Cu grids. • Polarized Optical Microscopy (POM): Nikon Optiphot-2, POL equipped with UFX-DX automatic exposure. Linkam THMS-600 with TP-92 temperature programmer was used as microscopic heating stage. NCKU-PRL Nation Cheng Kung Univ. Polym. Res. Lab.

Solution Intercalation of s. PS Solvent used - 1, 1, 2, 2 -tetrachloroethane (TCE) Solution was stirred for 1 h at 130 o. C to dissolve s. PS (2 -wt%) Clay (vacuum dried at 80 o. C for 6 h) was added into the polymer solution Clay suspension was vigorously stirred at 60 o. C in oil bath for 24 h The resultant solution was cast as thin film at 60 o. C for 24 h Residual solvent was removed by vacuum drying at 110 o. C for 7 days Cast film was grounded to fine powder Powdered samples were further dried at 110 o. C for 3 days for complete removal of solvent Nation Cheng Kung Univ. NCKU-PRL Polym. Res. Lab.

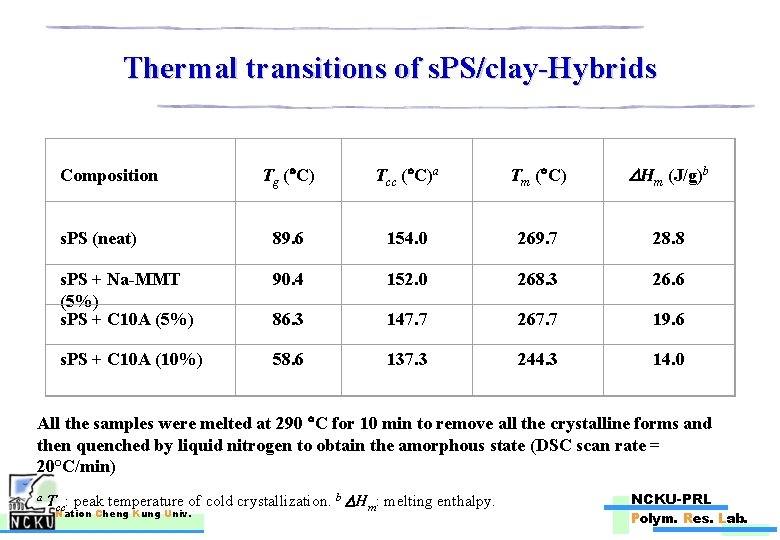
Thermal transitions of s. PS/clay-Hybrids Composition s. PS (neat) s. PS + Na-MMT (5%) s. PS + C 10 A (5%) Tg ( C) Tcc ( C)a Tm ( C) Hm (J/g)b 89. 6 154. 0 269. 7 28. 8 90. 4 152. 0 268. 3 26. 6 86. 3 147. 7 267. 7 19. 6 s. PS + C 10 A (10%) 58. 6 137. 3 244. 3 14. 0 All the samples were melted at 290 C for 10 min to remove all the crystalline forms and then quenched by liquid nitrogen to obtain the amorphous state (DSC scan rate = 20°C/min) a T cc: peak temperature of cold crystallization. b Hm: melting enthalpy. Nation Cheng Kung Univ. NCKU-PRL Polym. Res. Lab.

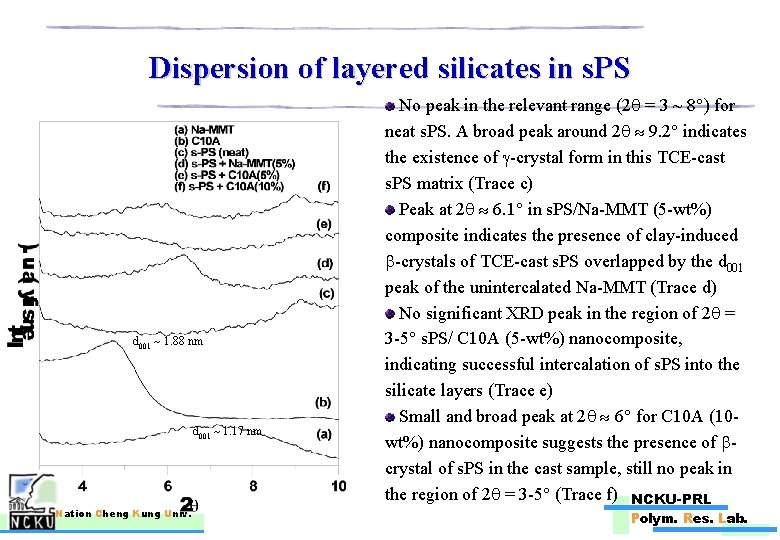
Dispersion of layered silicates in s. PS d 001 1. 88 nm d 001 1. 17 nm Nation Cheng Kung Univ. No peak in the relevant range (2 = 3 ~ 8 ) for neat s. PS. A broad peak around 2 9. 2 indicates the existence of -crystal form in this TCE-cast s. PS matrix (Trace c) Peak at 2 6. 1 in s. PS/Na-MMT (5 -wt%) composite indicates the presence of clay-induced -crystals of TCE-cast s. PS overlapped by the d 001 peak of the unintercalated Na-MMT (Trace d) No significant XRD peak in the region of 2 = 3 -5 s. PS/ C 10 A (5 -wt%) nanocomposite, indicating successful intercalation of s. PS into the silicate layers (Trace e) Small and broad peak at 2 6 for C 10 A (10 wt%) nanocomposite suggests the presence of crystal of s. PS in the cast sample, still no peak in the region of 2 = 3 -5 (Trace f) NCKU-PRL Polym. Res. Lab.

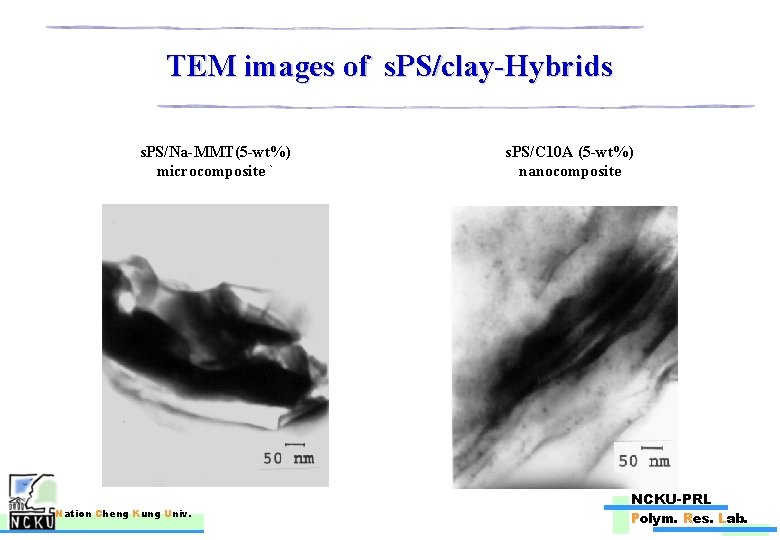
TEM images of s. PS/clay-Hybrids s. PS/Na-MMT(5 -wt%) microcomposite ` Nation Cheng Kung Univ. s. PS/C 10 A (5 -wt%) nanocomposite NCKU-PRL Polym. Res. Lab.

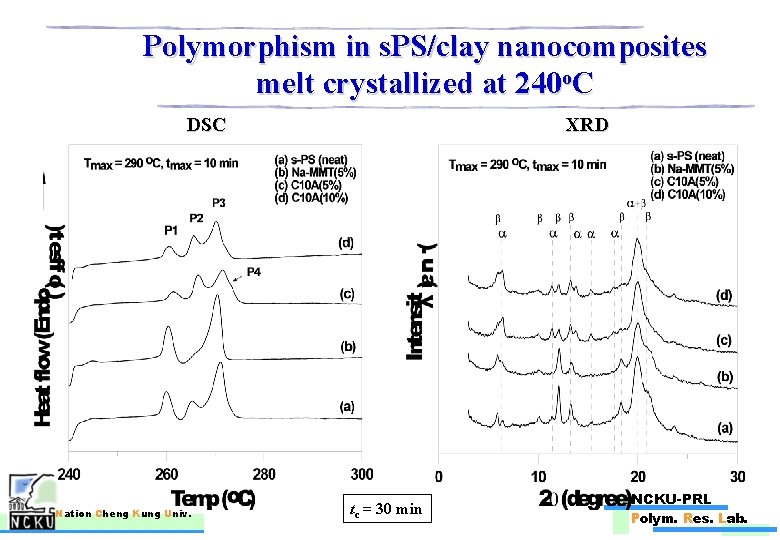
Polymorphism in s. PS/clay nanocomposites melt crystallized at 240 o. C DSC Nation Cheng Kung Univ. XRD tc = 30 min NCKU-PRL Polym. Res. Lab.

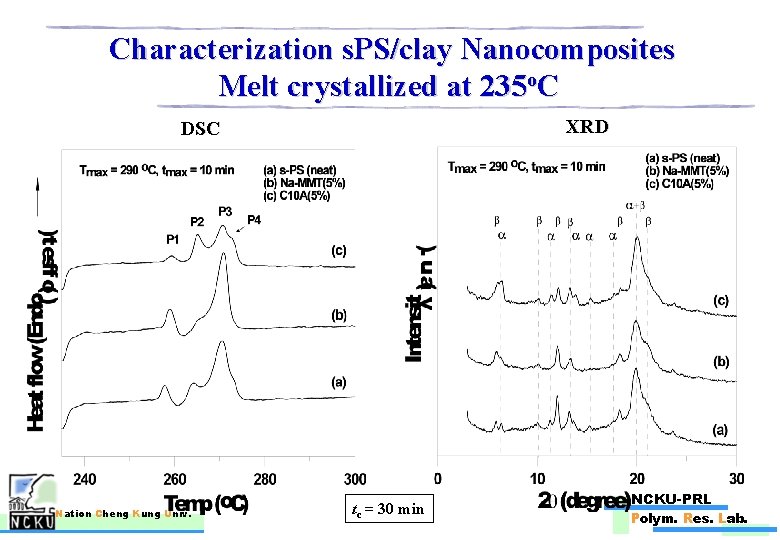
Characterization s. PS/clay Nanocomposites Melt crystallized at 235 o. C XRD DSC Nation Cheng Kung Univ. tc = 30 min NCKU-PRL Polym. Res. Lab.

Crystal forms in s. PS/clay nanocomposites melt crystallized at 245 o. C DSC Nation Cheng Kung Univ. XRD tc = 60 min NCKU-PRL Polym. Res. Lab.

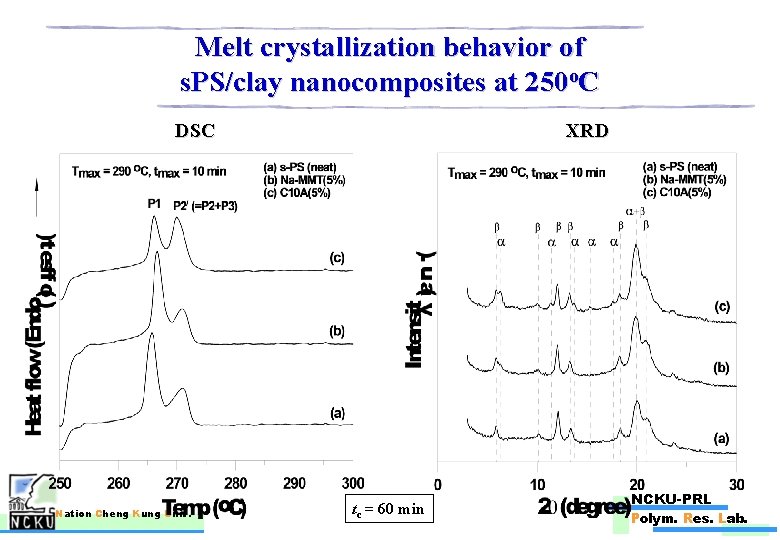
Melt crystallization behavior of s. PS/clay nanocomposites at 250 o. C DSC Nation Cheng Kung Univ. XRD tc = 60 min NCKU-PRL Polym. Res. Lab.

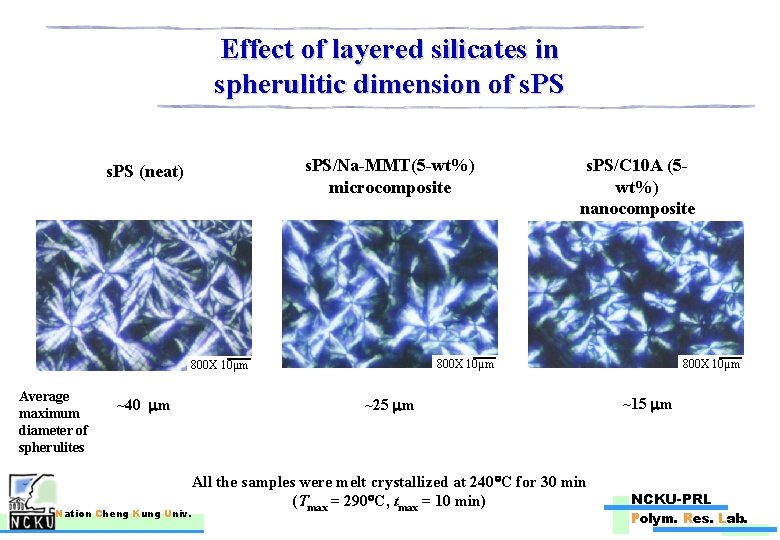
Effect of layered silicates in spherulitic dimension of s. PS/Na-MMT(5 -wt%) microcomposite s. PS (neat) 800 X 10μm Average maximum diameter of spherulites ~40 m Nation Cheng Kung Univ. s. PS/C 10 A (5 wt%) nanocomposite ~25 m All the samples were melt crystallized at 240 C for 30 min (Tmax = 290 C, tmax = 10 min) 800 X 10μm ~15 m NCKU-PRL Polym. Res. Lab.

Conclusions The s. PS molecules were successfully intercalated into the organo-clay galleries. TEM analysis revealed a mixed morphology of an intercalated/exfoliated structure in the s. PS/clay nanocomposites. A significant alteration of the polymorphism in the s. PS matrix was observed by the inclusion of different nano-layered clays. The temperature regime of the -crystal formation in s. PS was found to expand considerably and the organo-clays favored the formation of -phase in addition to the -phase, even at high Tc of 250 C. Pristine clay (Na-MMT) was dispersed in the s. PS matrix more coarsely with aggregated structures, behaved differently in its nucleating action and only larger spherulites of form of s. PS crystals were induced to grow at all the available Tc’s. the clay dispersibility in the s. PS matrix is assumed to play a pivotal role in altering the crystalline structures. The inclusion of the organo-clay with nano-scale dispersibility promoted the rapid formation of -forms, which developed spherulites of smaller dimension as compared the -forms. However, the proportion of -phase was decreased with higher loading (> 5 -wt%) of organo-clay due to poor dispersion. The peak 2 (P 2) for melt-crystallized s. PS in DSC analysis was further confirmed to correspond to the -phase, as it was almost absent in the s. PS/Na-MMT composites. Nation Cheng Kung Univ. NCKU-PRL Polym. Res. Lab.

Polymorphic Crystal Forms and Melting Behavior of Poly(butylene adipate) Nation Cheng Kung Univ. NCKU-PRL Polym. Res. Lab.

Introduction • Polymorphic structure of Poly(butylene adipate)(PBA) was reported by Minke and Blackwell in 1979. They have identified two types of polymorphic crystals exhibited in crystalline PBA samples. a-from crystal was characterized as a monoclinic unit cell, and -form was packed as an orthorhombicunit cell. • For PBA, in the previous paper, it was indicated that the formation of a- and -form crystal was dependent on the melt crystallization temperatures. • In this study, we attempted to explore the relationships between the polymorphic crystals and the multiple melting peaks. Nation Cheng Kung Univ. NCKU-PRL Polym. Res. Lab.

Experimental Material Used Poly(butylene adipate)(PBA) (Aldrich Chem. Co. ) Mw=12, 200 and Mn=9, 540 with a polydispersity of 1. 28. • Purification: PBA was reprecipitated from chloroform into cold methanol (ca. – 10 o. C). And then, the sample was dried in the vacuum oven at 40 o. C for 7 days to remove the solvent. Nation Cheng Kung Univ. NCKU-PRL Polym. Res. Lab.

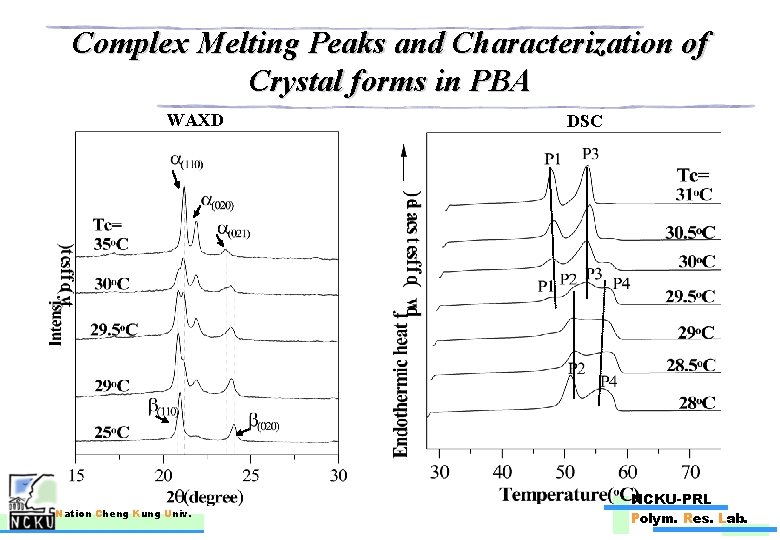
Complex Melting Peaks and Characterization of Crystal forms in PBA WAXD Nation Cheng Kung Univ. DSC NCKU-PRL Polym. Res. Lab.

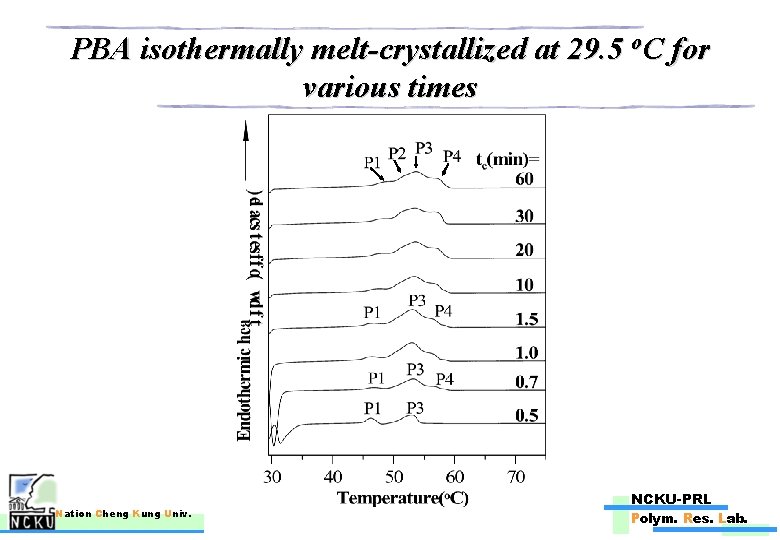
PBA isothermally melt-crystallized at 29. 5 o. C for various times Nation Cheng Kung Univ. NCKU-PRL Polym. Res. Lab.

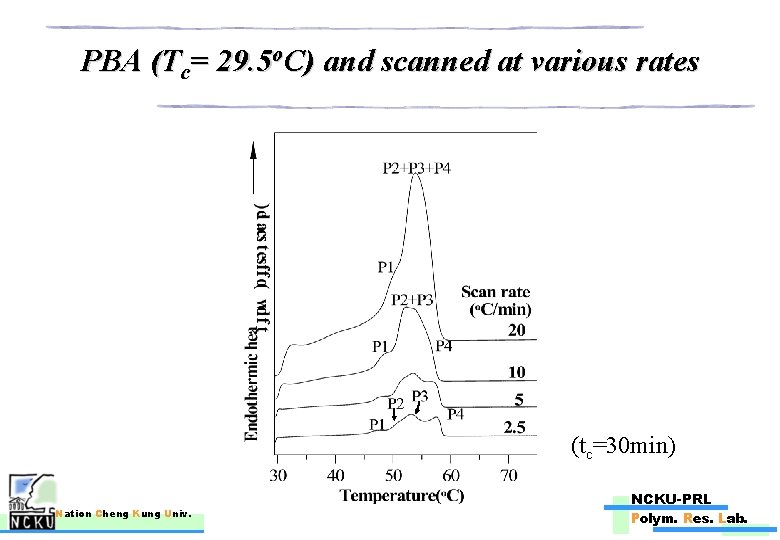
PBA (Tc= 29. 5 o. C) and scanned at various rates (tc=30 min) Nation Cheng Kung Univ. NCKU-PRL Polym. Res. Lab.

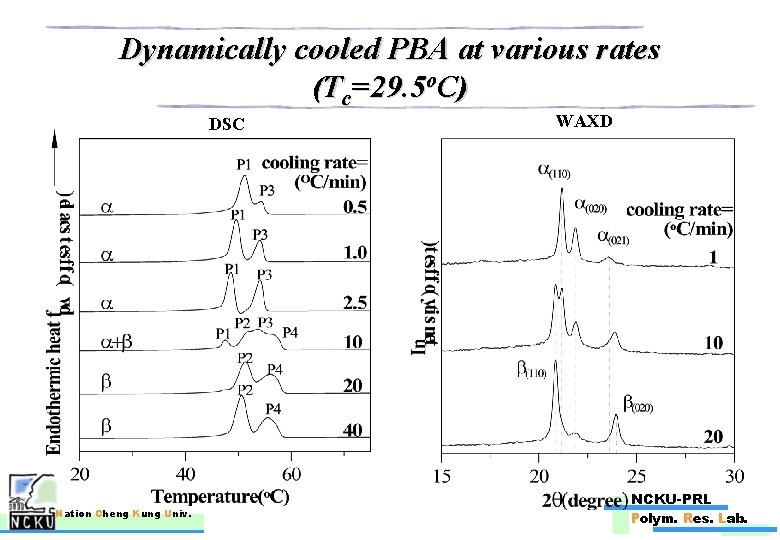
Dynamically cooled PBA at various rates (Tc=29. 5 o. C) DSC Nation Cheng Kung Univ. WAXD NCKU-PRL Polym. Res. Lab.

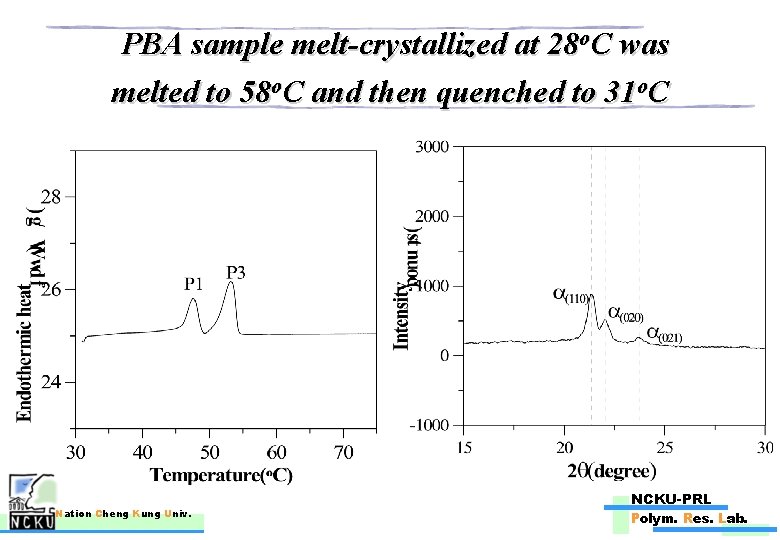
PBA sample melt-crystallized at 28 o. C was melted to 58 o. C and then quenched to 31 o. C Nation Cheng Kung Univ. NCKU-PRL Polym. Res. Lab.

PBA (Tc=31 o. C) melted to different Tm and then quenched to 28 o. C Nation Cheng Kung Univ. NCKU-PRL Polym. Res. Lab.

Comparison of the melting behaviors of aform crystal melted to various temperatures Nation Cheng Kung Univ. NCKU-PRL Polym. Res. Lab.

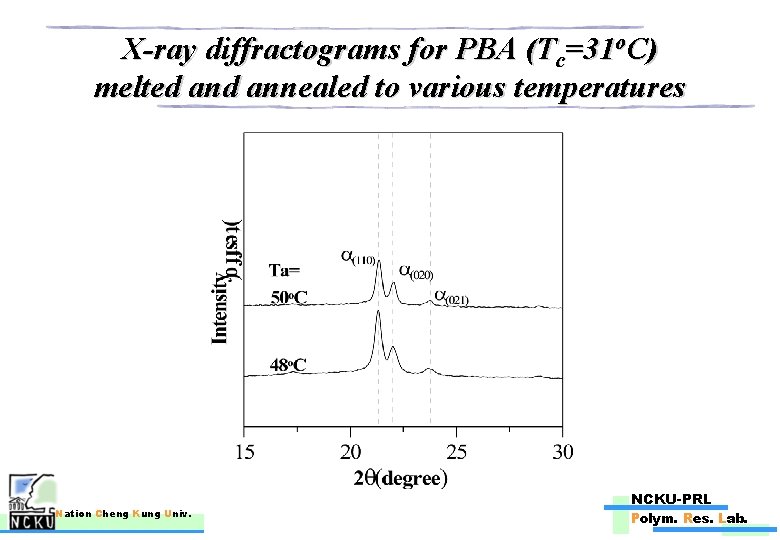
X-ray diffractograms for PBA (Tc=31 o. C) melted annealed to various temperatures Nation Cheng Kung Univ. NCKU-PRL Polym. Res. Lab.

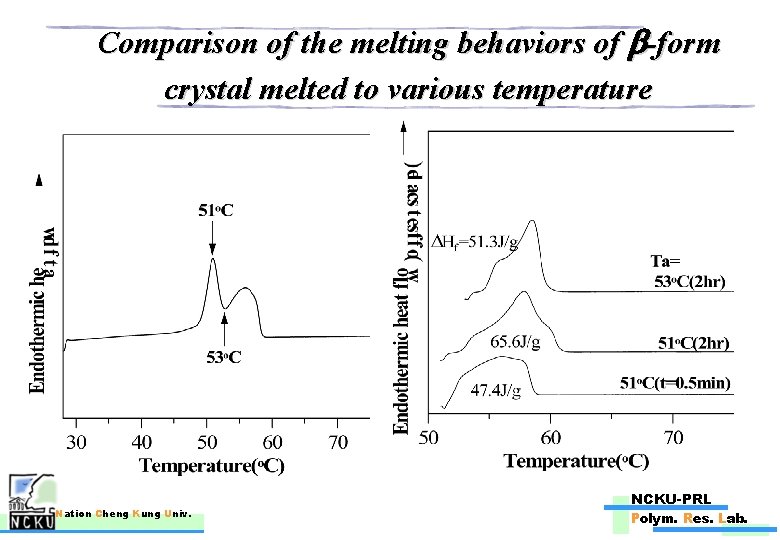
Comparison of the melting behaviors of b-form crystal melted to various temperature Nation Cheng Kung Univ. NCKU-PRL Polym. Res. Lab.

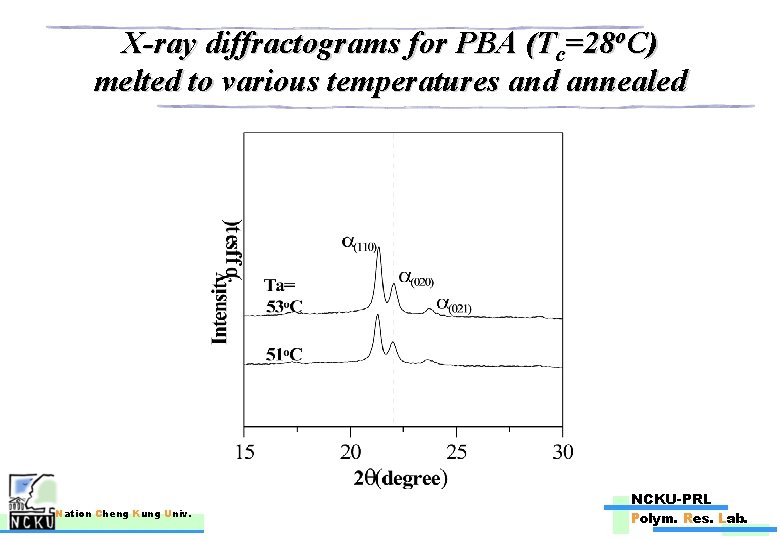
X-ray diffractograms for PBA (Tc=28 o. C) melted to various temperatures and annealed Nation Cheng Kung Univ. NCKU-PRL Polym. Res. Lab.

Conclusion • The formation of -form crystal can be favored in the crystallization of PBA under these two conditions: (1) slow cooling from the molten state, (2) melt crystallization at high temperatures. • The -form crystal can be formed under these two conditions: (1) melt crystallization at lower temperatures, (2) at fast cooling rates from the melt. • The -form crystal which was heated to melt produced two endothermic peaks (P 1 and P 3). P 2 and P 4 appeared when -from crystal was melted. As - and -form crystals coexisted, the melting behaviors for the crystals showed four peaks (P 1 -P 4) on the DSC trace. Nation Cheng Kung Univ. NCKU-PRL Polym. Res. Lab.

Part 1 Miscibility in Ternary Blends: PVAc/PVPh/PMMA Nation Cheng Kung Univ. NCKU-PRL Polym. Res. Lab.

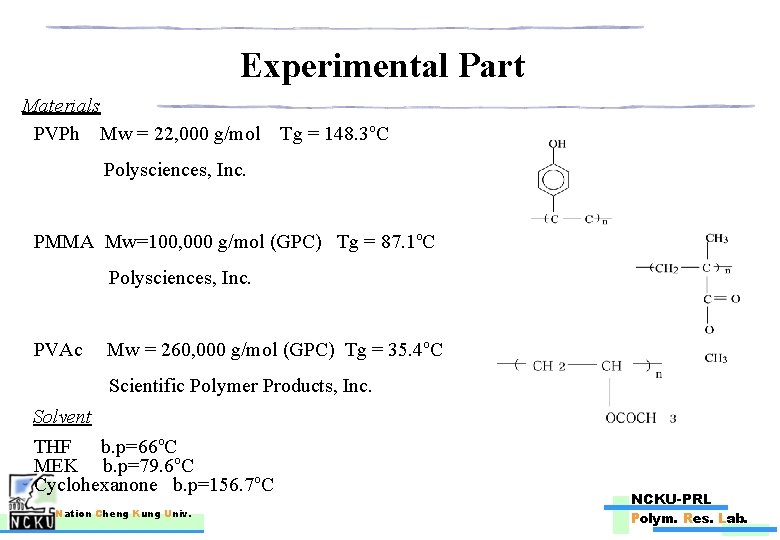
Experimental Part Materials PVPh Mw = 22, 000 g/mol Tg = 148. 3 o. C Polysciences, Inc. PMMA Mw=100, 000 g/mol (GPC) Tg = 87. 1 o. C Polysciences, Inc. PVAc Mw = 260, 000 g/mol (GPC) Tg = 35. 4 o. C Scientific Polymer Products, Inc. Solvent THF b. p=66 o. C MEK b. p=79. 6 o. C Cyclohexanone b. p=156. 7 o. C Nation Cheng Kung Univ. NCKU-PRL Polym. Res. Lab.

Experimental Part(Contd. ) Sample Preparation The blend samples were prepared by solvent-casting (THF, MEK and cyclohexanone). When completely mixing that The blend samples were cast at 45 o. C for 24 hr. Samples were subjected to vacuum degassing at 80 o. C for one week Apparatus Optical light microscope (Nikon Optiphot-2, POL) Different scanning calorimeter (Perkin-Elmer DSC-7) Scanning electron microscope (SEM, JEOL JXA 840) Nation Cheng Kung Univ. NCKU-PRL Polym. Res. Lab.

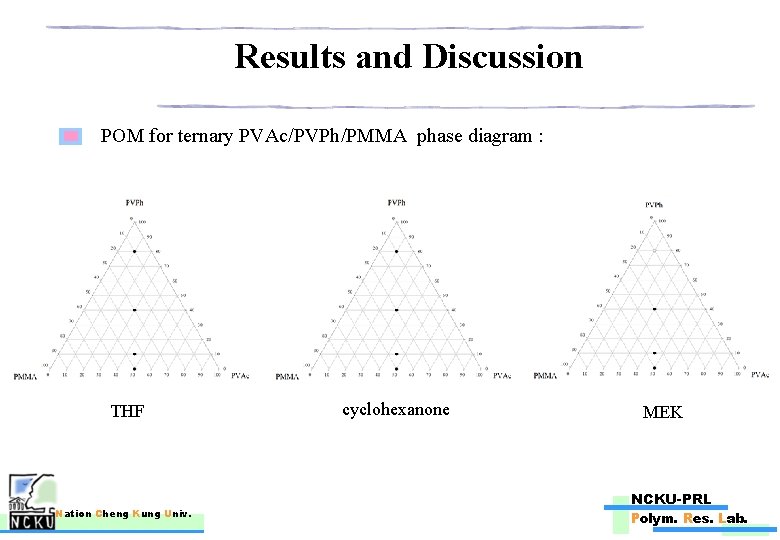
Results and Discussion POM for ternary PVAc/PVPh/PMMA phase diagram : THF Nation Cheng Kung Univ. cyclohexanone MEK NCKU-PRL Polym. Res. Lab.

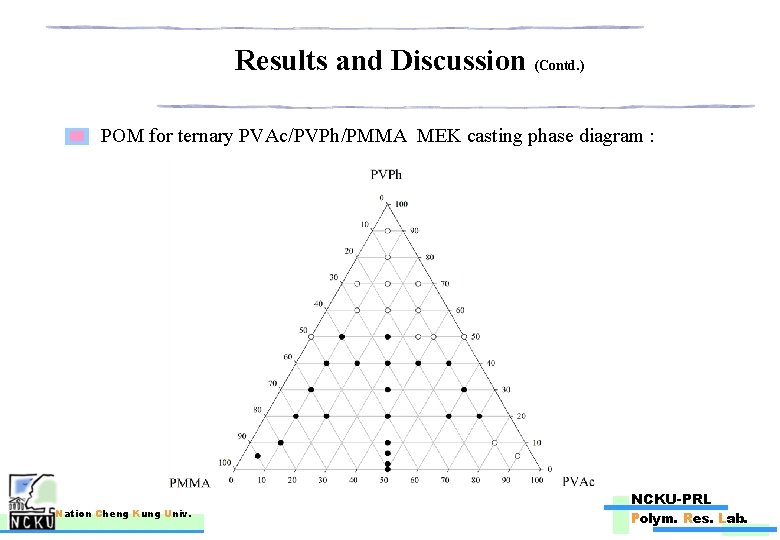
Results and Discussion (Contd. ) POM for ternary PVAc/PVPh/PMMA MEK casting phase diagram : Nation Cheng Kung Univ. NCKU-PRL Polym. Res. Lab.

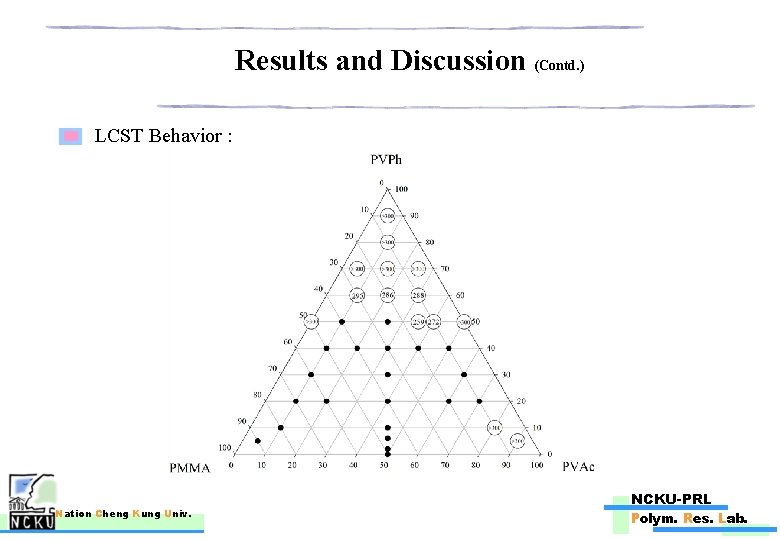
Results and Discussion (Contd. ) LCST Behavior : Nation Cheng Kung Univ. NCKU-PRL Polym. Res. Lab.

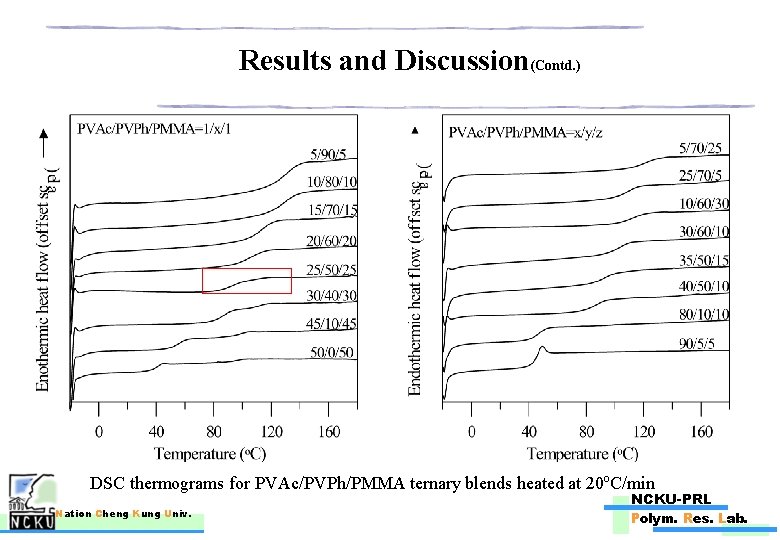
Results and Discussion(Contd. ) DSC thermograms for PVAc/PVPh/PMMA ternary blends heated at 20 o. C/min Nation Cheng Kung Univ. NCKU-PRL Polym. Res. Lab.

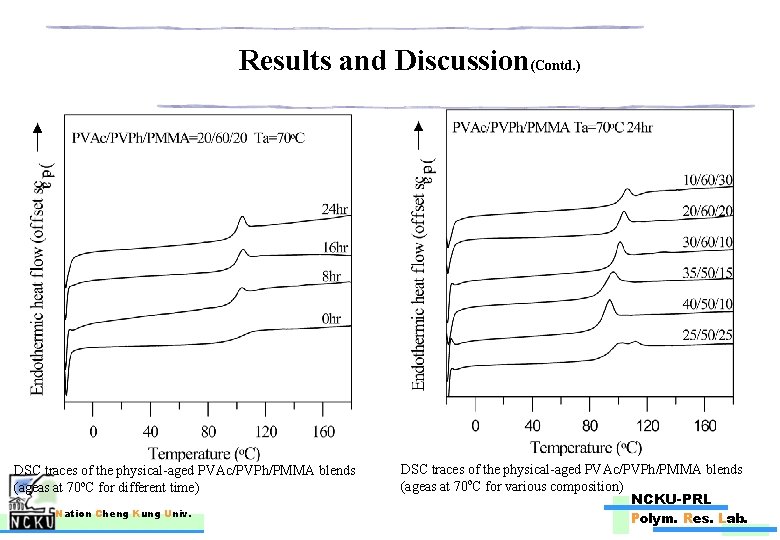
Results and Discussion(Contd. ) DSC traces of the physical-aged PVAc/PVPh/PMMA blends (ageas at 70 o. C for different time) Nation Cheng Kung Univ. DSC traces of the physical-aged PVAc/PVPh/PMMA blends (ageas at 70 o. C for various composition) NCKU-PRL Polym. Res. Lab.

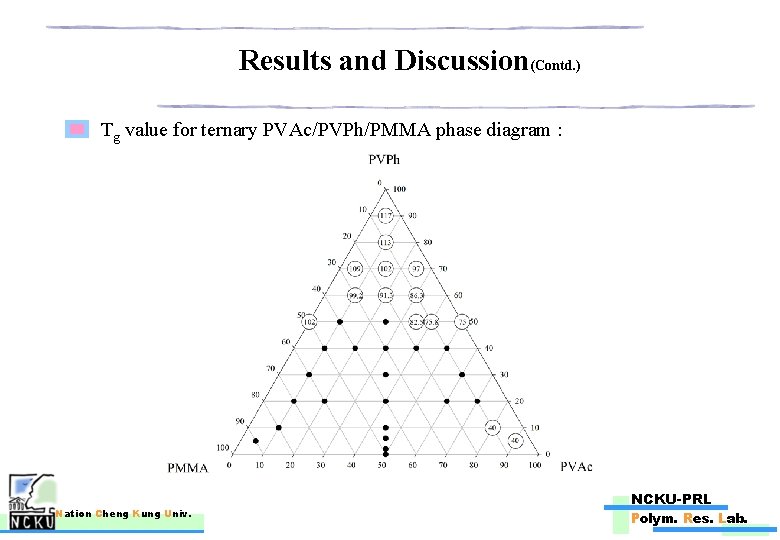
Results and Discussion(Contd. ) Tg value for ternary PVAc/PVPh/PMMA phase diagram : Nation Cheng Kung Univ. NCKU-PRL Polym. Res. Lab.

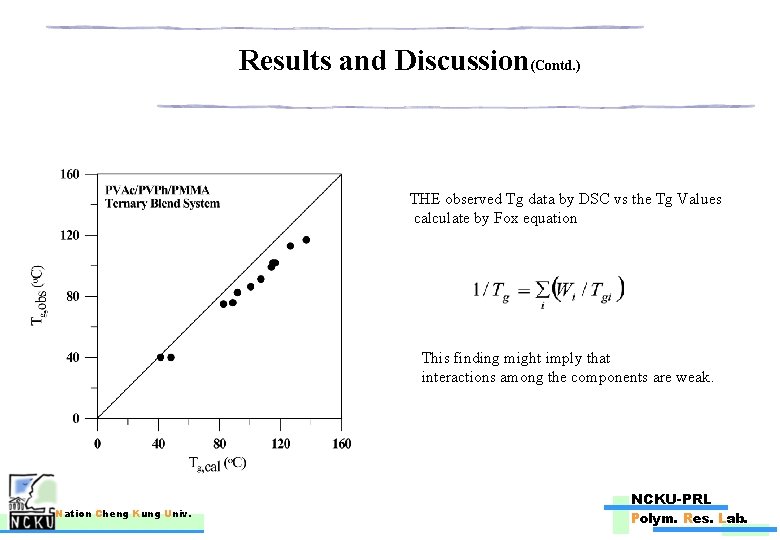
Results and Discussion(Contd. ) THE observed Tg data by DSC vs the Tg Values calculate by Fox equation This finding might imply that interactions among the components are weak. Nation Cheng Kung Univ. NCKU-PRL Polym. Res. Lab.

15/70/15 10/80/10 5/90/5 30/60/10 10/60/30 20/60/20 80/10/10 8000 X 5000 X 40/50/10 5000 X 6000 X 8000 X 35/50/15 1800 X SEM micrographs of PVAc/PVPh/PMMA ternary blends of virious compositions. Nation Cheng Kung Univ. 5000 X NCKU-PRL Polym. Res. Lab.

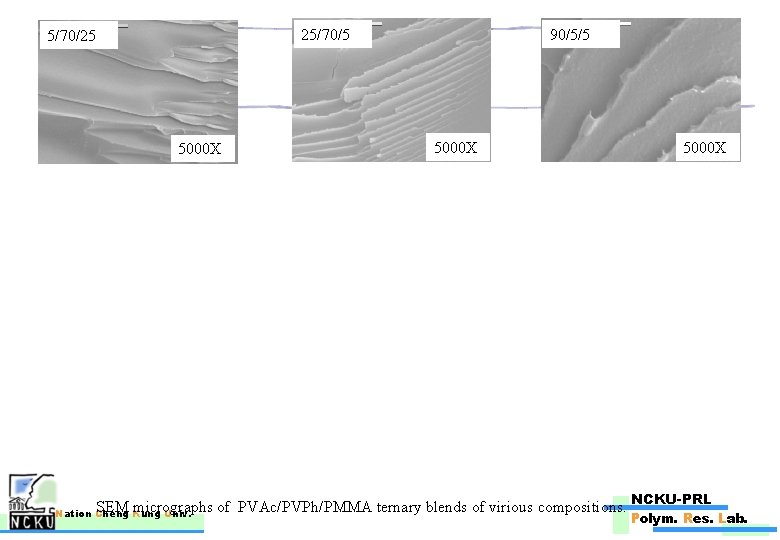
25/70/5 5/70/25 5000 X 90/5/5 5000 X SEM micrographs of PVAc/PVPh/PMMA ternary blends of virious compositions. Nation Cheng Kung Univ. 5000 X NCKU-PRL Polym. Res. Lab.

Part 2 LPVAc/PVPh/LPMMA Nation Cheng Kung Univ. NCKU-PRL Polym. Res. Lab.

Experimental Part Materials PVPh Mw = 22, 000 g/mol Tg = 148. 3 o. C Polysciences, Inc. LPMMA Mw=15, 000 g/mol (GPC) Tg = 75. 8 o. C Aldrich Chemical Company, Inc. LPVAc Mw = 12, 800 g/mol (GPC) Tg = 31. 3 o. C Aldrich Chemical Company, Inc. solvent MEK b. p=79. 6 o. C Nation Cheng Kung Univ. NCKU-PRL Polym. Res. Lab.

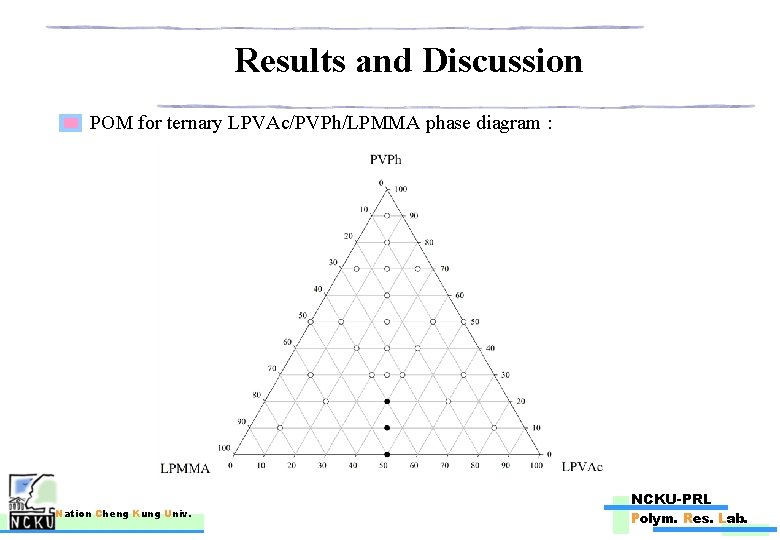
Results and Discussion POM for ternary LPVAc/PVPh/LPMMA phase diagram : Nation Cheng Kung Univ. NCKU-PRL Polym. Res. Lab.

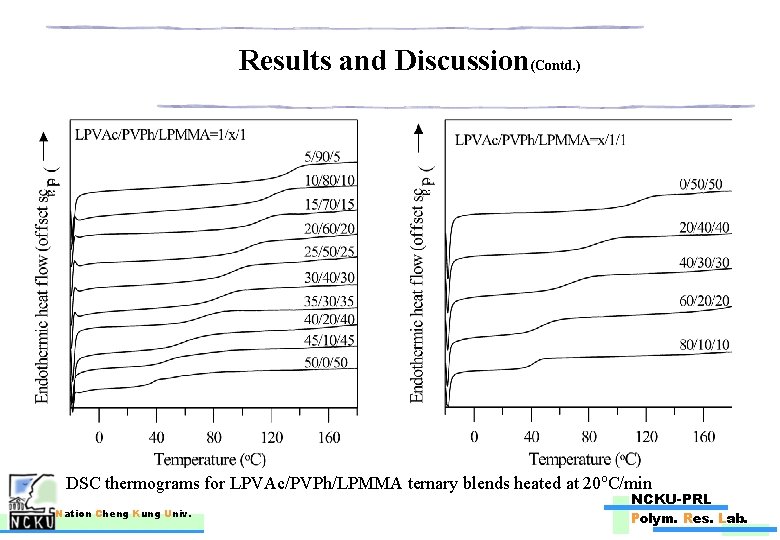
Results and Discussion(Contd. ) DSC thermograms for LPVAc/PVPh/LPMMA ternary blends heated at 20 o. C/min Nation Cheng Kung Univ. NCKU-PRL Polym. Res. Lab.

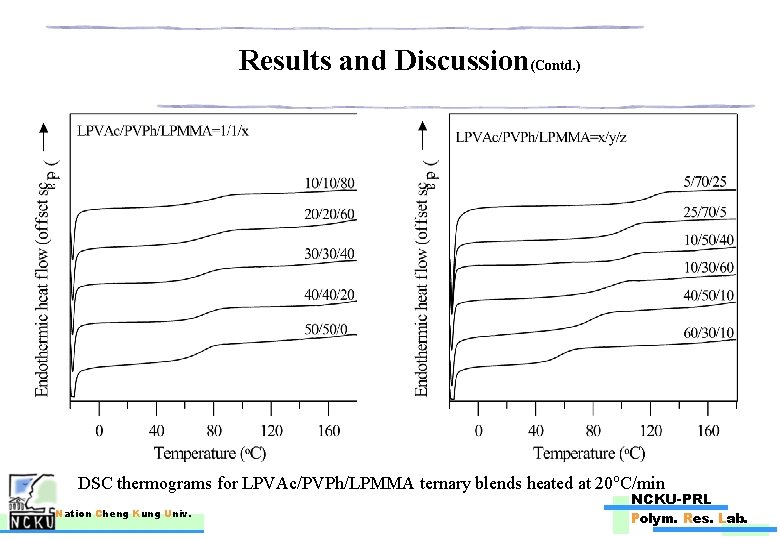
Results and Discussion(Contd. ) DSC thermograms for LPVAc/PVPh/LPMMA ternary blends heated at 20 o. C/min Nation Cheng Kung Univ. NCKU-PRL Polym. Res. Lab.

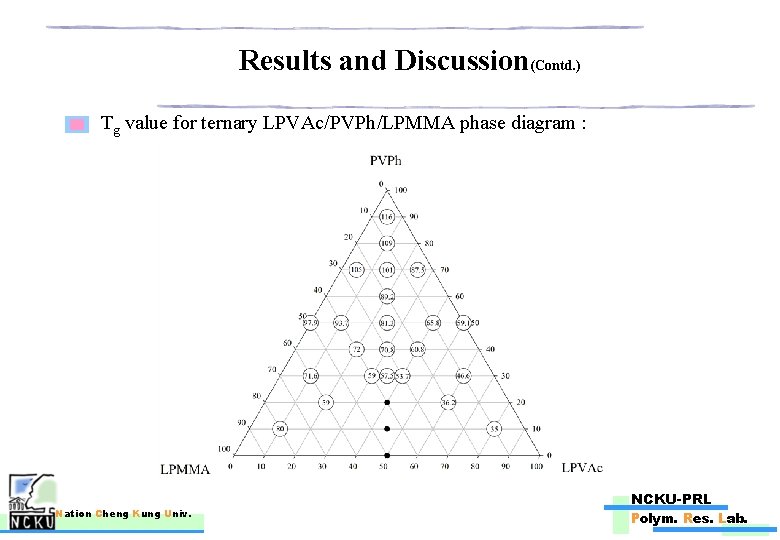
Results and Discussion(Contd. ) Tg value for ternary LPVAc/PVPh/LPMMA phase diagram : Nation Cheng Kung Univ. NCKU-PRL Polym. Res. Lab.

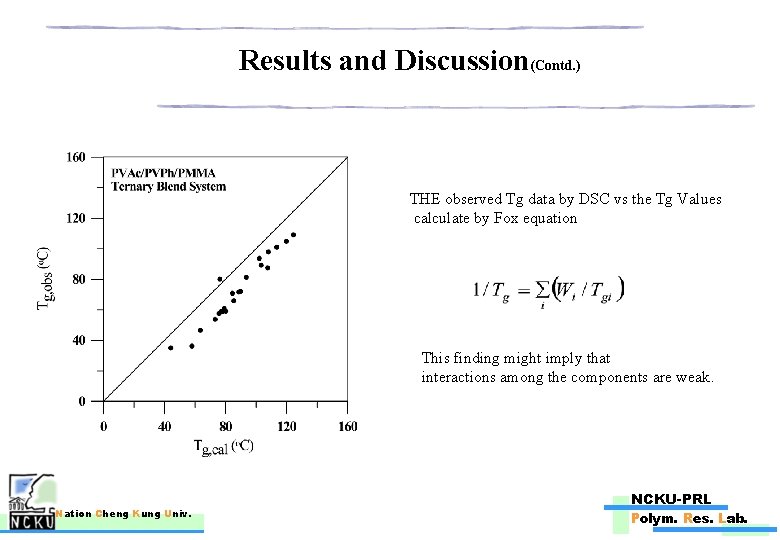
Results and Discussion(Contd. ) THE observed Tg data by DSC vs the Tg Values calculate by Fox equation This finding might imply that interactions among the components are weak. Nation Cheng Kung Univ. NCKU-PRL Polym. Res. Lab.

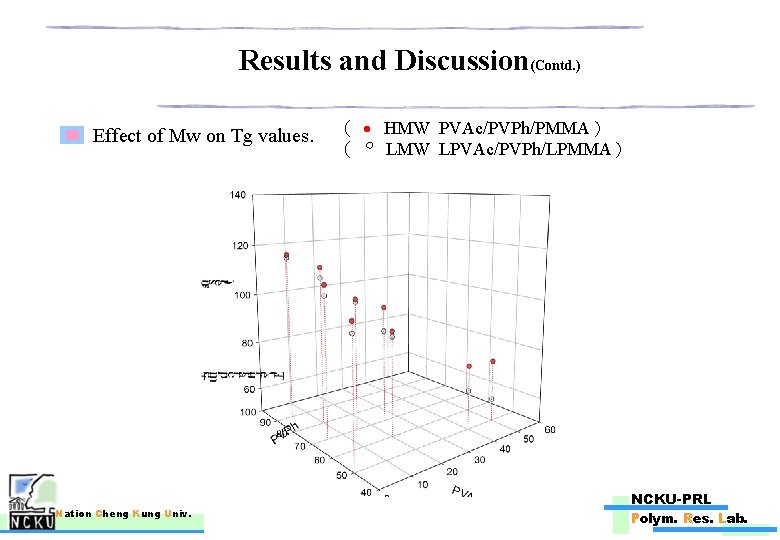
Results and Discussion(Contd. ) Effect of Mw on Tg values. Nation Cheng Kung Univ. ( HMW PVAc/PVPh/PMMA) ( LMW LPVAc/PVPh/LPMMA) 。 NCKU-PRL Polym. Res. Lab.

Part 3 LPVAc/PVPh/LPMMA Cyclohexanone solvent and co-precipitated Nation Cheng Kung Univ. NCKU-PRL Polym. Res. Lab.

Experimental Part Sample Preparation The blend samples were prepared by solvent-casting (cyclohexanone). When completely mixing that the blend samples were cast at 50 o. C and 90 o. C for 24 hr. Samples were subjected to vacuum degassing at 80 o. C for one week Apparatus Optical light microscope (Nikon Optiphot-2, POL) Nation Cheng Kung Univ. NCKU-PRL Polym. Res. Lab.

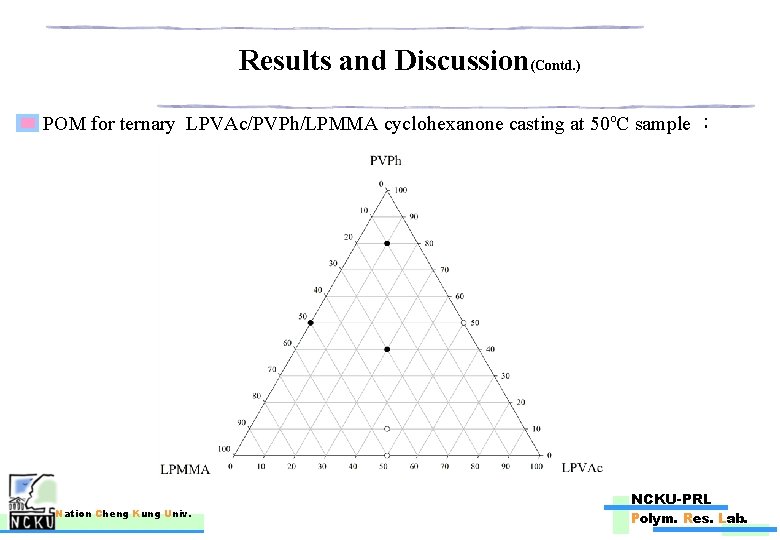
Results and Discussion(Contd. ) POM for ternary LPVAc/PVPh/LPMMA cyclohexanone casting at 50 o. C sample : Nation Cheng Kung Univ. NCKU-PRL Polym. Res. Lab.

Results and Discussion(Contd. ) POM for ternary LPVAc/PVPh/LPMMA cyclohexanone casting at 90 o. C sample : Nation Cheng Kung Univ. NCKU-PRL Polym. Res. Lab.

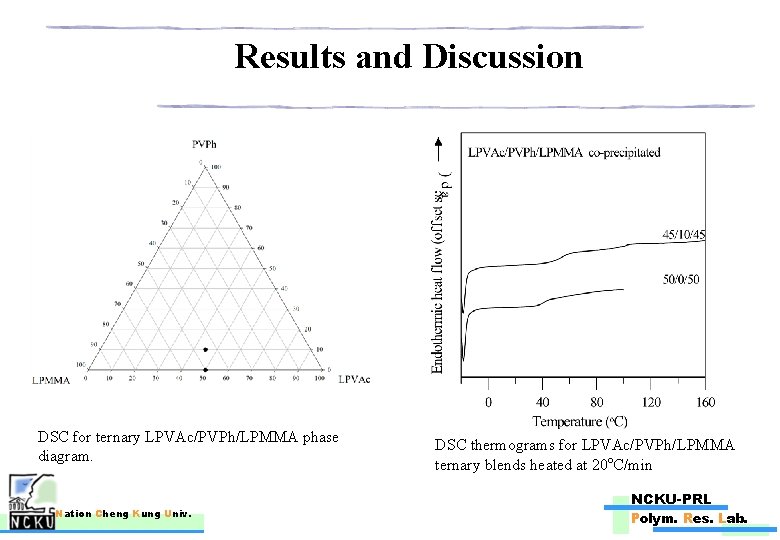
Results and Discussion DSC for ternary LPVAc/PVPh/LPMMA phase diagram. Nation Cheng Kung Univ. DSC thermograms for LPVAc/PVPh/LPMMA ternary blends heated at 20 o. C/min NCKU-PRL Polym. Res. Lab.

Conclusion In this ternary blend of PVAc/PVPh/PMMA where PVPh is miscible with each of the other component, more than 50 wt% PVPh was required to cause miscibility between PVAc and PMMA. Phase boundary of LPVAc/PVPh/LPMMA ternary blend investigated by DSC shows that miscibility window was increased obviously. Furthermor the Tg value of LPVAc/PVPh/LPMMA was less than PVAc/PVPh/PMMA system. Blend prepared by cyclohexanone solvent casting and co-precipitated (MEK-hexane) were found to be immiscible. Nation Cheng Kung Univ. NCKU-PRL Polym. Res. Lab.

Chiang Chih Pei Nation Cheng Kung Univ. NCKU-PRL Polym. Res. Lab.

System 1. The blend of Poly(p-vinylphenol) (PVPh) / Poly(1, 6 -hexamethylene adipate)(PHA) 2. The blend of Poly(p-vinylphenol) (PVPh) / Poly(ethylene azelate)(PEAz) 3. The blend of Poly(p-vinylphenol) (PVPh) / Poly(hexamethylene sebacate)(PHS) Nation Cheng Kung Univ. NCKU-PRL Polym. Res. Lab.

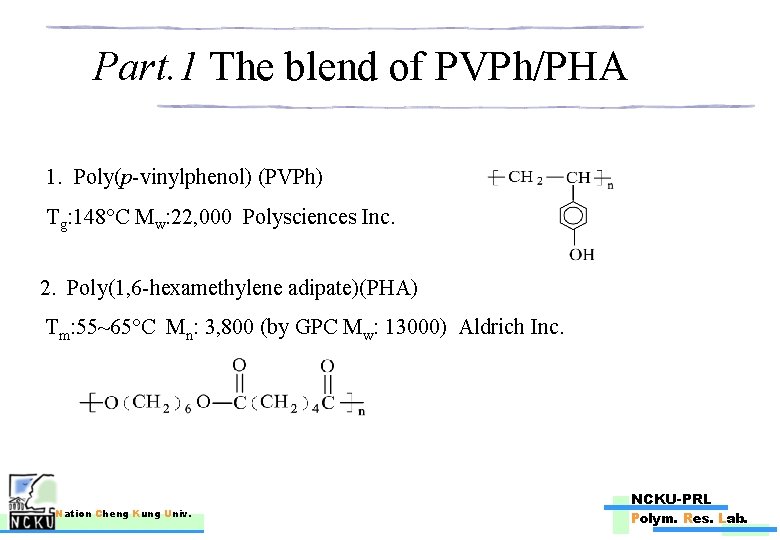
Part. 1 The blend of PVPh/PHA 1. Poly(p-vinylphenol) (PVPh) Tg: 148°C Mw: 22, 000 Polysciences Inc. 2. Poly(1, 6 -hexamethylene adipate)(PHA) Tm: 55~65°C Mn: 3, 800 (by GPC Mw: 13000) Aldrich Inc. Nation Cheng Kung Univ. NCKU-PRL Polym. Res. Lab.

Methods Sample preparation All polymers blends via solution-casting. Solutions were prepared in tetrahydrofuran (THF) at a concentration of 4 g polymer/ 100 m. L solvent to obtain clear solution mixtures at room temperature; then mixtures were cast at 45°C onto hot stage for 24 hr. The solution-cast films were dried for a week in 40°C vacuum oven. Apparatus Polarizing optical microscope, POM Differential scanning calorimeter, DSC Nation Cheng Kung Univ. NCKU-PRL Polym. Res. Lab.

DSC traces of the PVPh/PHA blends (2 nd run) Nation Cheng Kung Univ. NCKU-PRL Polym. Res. Lab.

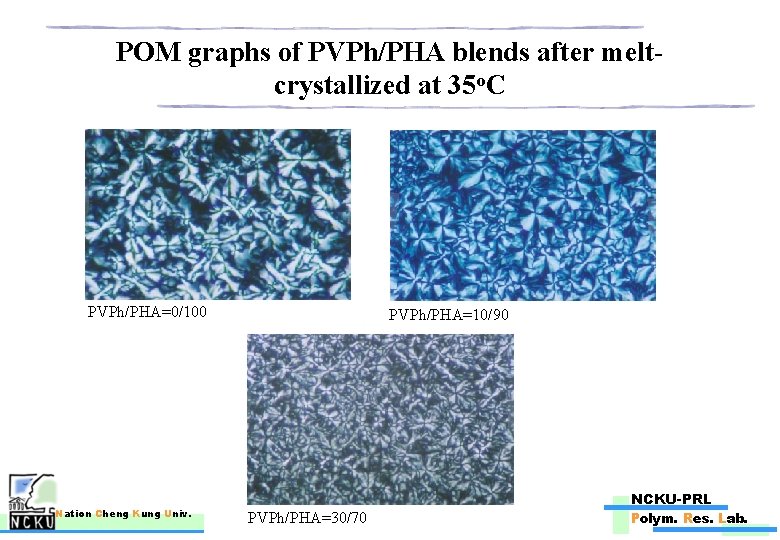
POM graphs of PVPh/PHA blends after meltcrystallized at 30 o. C PVPh/PHA=10/90 PVPh/PHA=0/100 Nation Cheng Kung Univ. NCKU-PRL PVPh/PHA=30/70 Polym. Res. Lab.

POM graphs of PVPh/PHA blends after meltcrystallized at 35 o. C PVPh/PHA=0/100 Nation Cheng Kung Univ. PVPh/PHA=10/90 NCKU-PRL PVPh/PHA=30/70 Polym. Res. Lab.

POM graphs of PVPh/PHA blends after meltcrystallized at 40 o. C PVPh/PHA=0/100 Nation Cheng Kung Univ. PVPh/PHA=10/90 PVPh/PHA=30/70 NCKU-PRL Polym. Res. Lab.

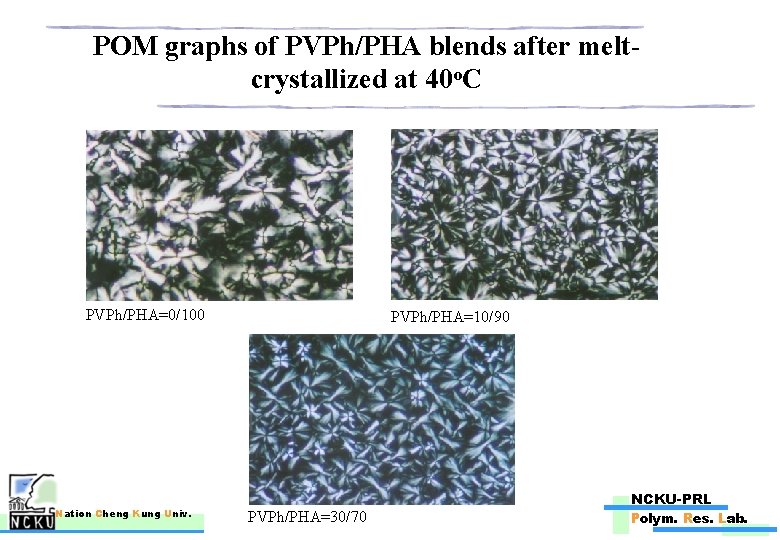
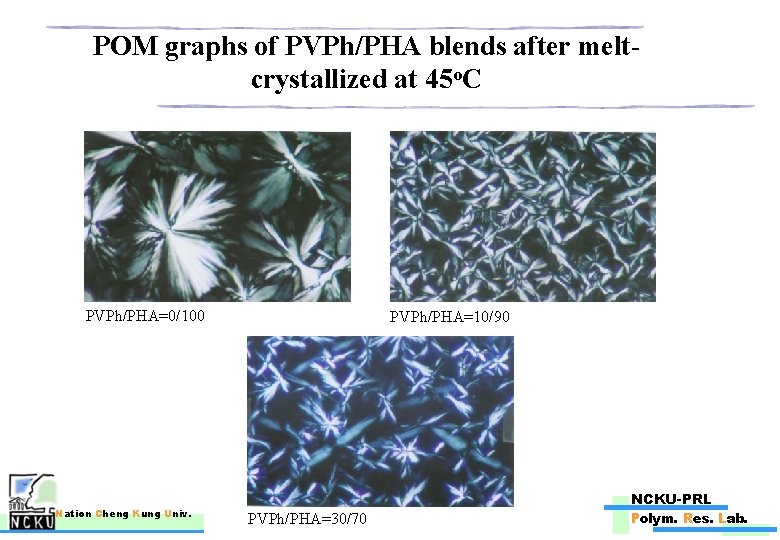
POM graphs of PVPh/PHA blends after meltcrystallized at 45 o. C PVPh/PHA=0/100 Nation Cheng Kung Univ. PVPh/PHA=10/90 NCKU-PRL PVPh/PHA=30/70 Polym. Res. Lab.

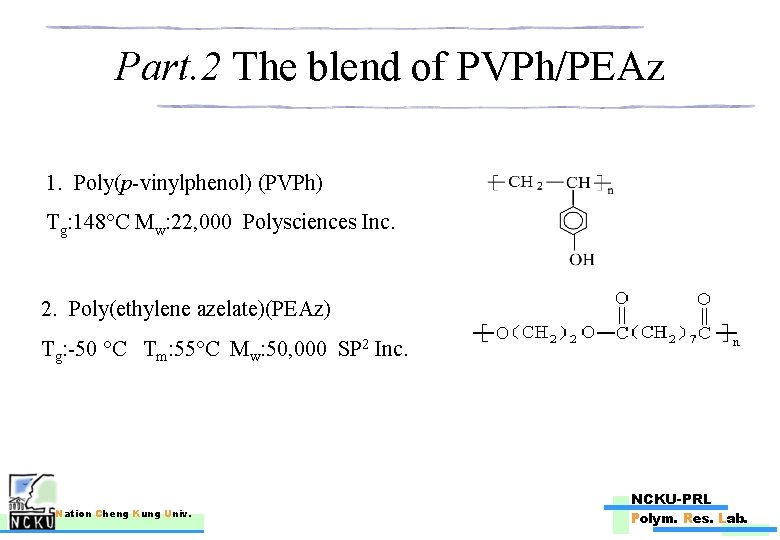
Part. 2 The blend of PVPh/PEAz 1. Poly(p-vinylphenol) (PVPh) Tg: 148°C Mw: 22, 000 Polysciences Inc. 2. Poly(ethylene azelate)(PEAz) Tg: -50 °C Tm: 55°C Mw: 50, 000 SP 2 Inc. Nation Cheng Kung Univ. NCKU-PRL Polym. Res. Lab.

Methods Sample preparation All polymers blends via solution-casting. Solutions were prepared in tetrahydrofuran (THF) at a concentration of 4 g polymer/ 100 m. L solvent to obtain clear solution mixtures at room temperature; then mixtures were cast at 45°C onto hot stage for 24 hr. The solution-cast films were dried for a week in 40°C vacuum oven. Apparatus Polarizing optical microscope, POM Differential scanning calorimeter, DSC Nation Cheng Kung Univ. NCKU-PRL Polym. Res. Lab.

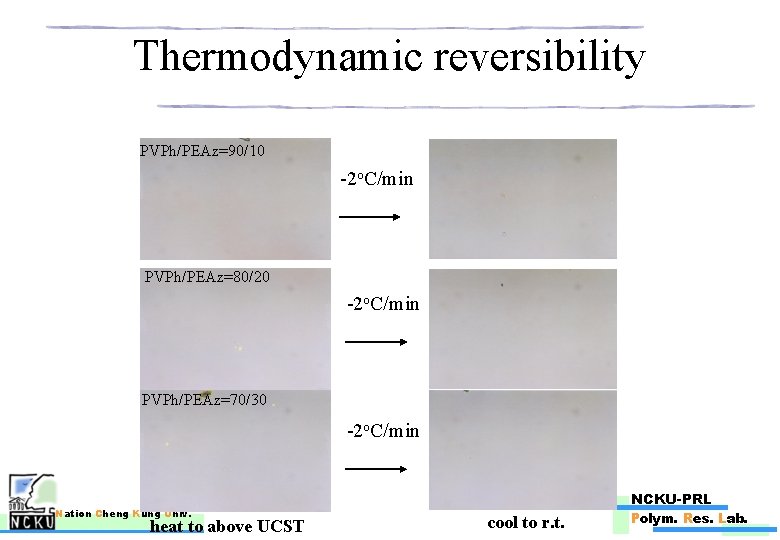
Thermodynamic reversibility PVPh/PEAz=90/10 -2 o. C/min PVPh/PEAz=80/20 -2 o. C/min PVPh/PEAz=70/30 -2 o. C/min Nation Cheng Kung Univ. heat to above UCST NCKU-PRL cool to r. t. Polym. Res. Lab.

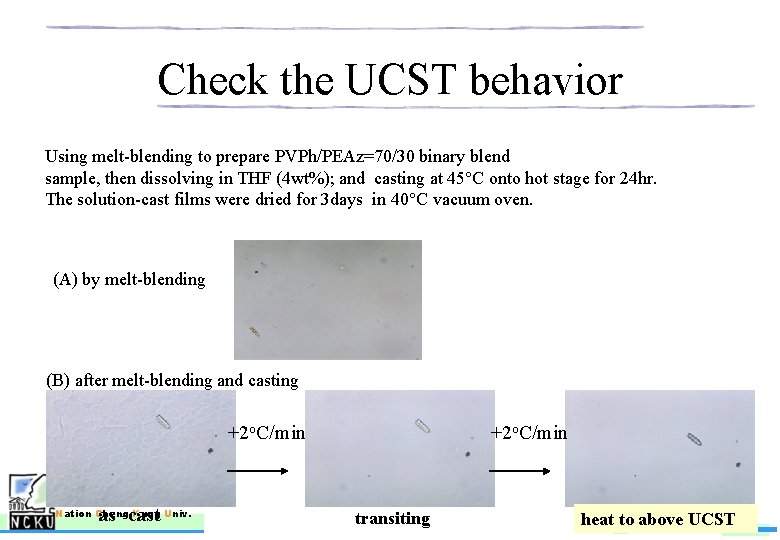
Check the UCST behavior Using melt-blending to prepare PVPh/PEAz=70/30 binary blend sample, then dissolving in THF (4 wt%); and casting at 45°C onto hot stage for 24 hr. The solution-cast films were dried for 3 days in 40°C vacuum oven. (A) by melt-blending (B) after melt-blending and casting +2 o. C/min as -cast Nation Cheng Kung Univ. +2 o. C/min transiting NCKU-PRL Polym. Res. Lab. heat to above UCST

Clarity point of the PVPh/PEAz blends one phase UCST one phase Nation Cheng Kung Univ. phase separation NCKU-PRL Polym. Res. Lab.

DSC traces of the PVPh/PEAz blends casting at 45 o. C Nation Cheng Kung Univ. NCKU-PRL Polym. Res. Lab.

DSC traces of the PVPh/PEAz blends after quenching from 200 o. C Nation Cheng Kung Univ. NCKU-PRL Polym. Res. Lab.

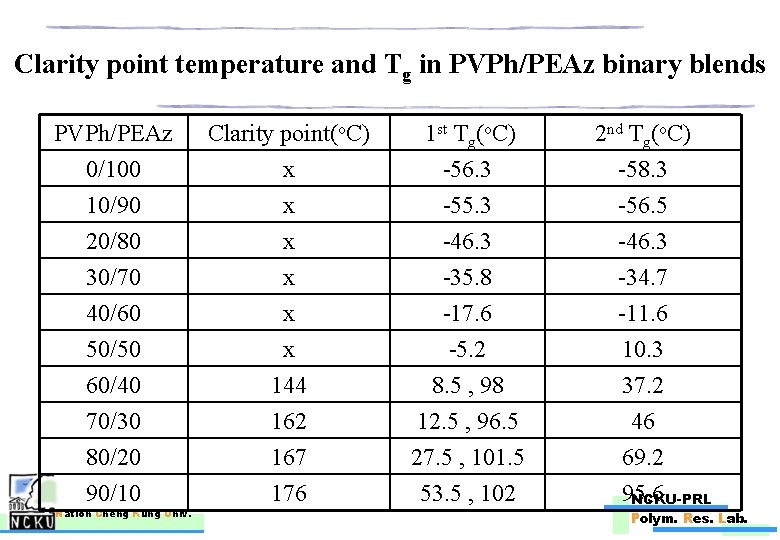
Clarity point temperature and Tg in PVPh/PEAz binary blends PVPh/PEAz 0/100 10/90 20/80 Clarity point(o. C) x x x 1 st Tg(o. C) -56. 3 -55. 3 -46. 3 30/70 40/60 50/50 60/40 70/30 80/20 90/10 x x x 144 162 167 176 -35. 8 -17. 6 -5. 2 8. 5 , 98 12. 5 , 96. 5 27. 5 , 101. 5 53. 5 , 102 Nation Cheng Kung Univ. 2 nd Tg(o. C) -58. 3 -56. 5 -46. 3 -34. 7 -11. 6 10. 3 37. 2 46 69. 2 95. 6 NCKU-PRL Polym. Res. Lab.
 Res. 1102-404 y res. 785
Res. 1102-404 y res. 785 La quinta declinazione latino
La quinta declinazione latino Res cogitans e res extensa
Res cogitans e res extensa What is chi kung fu panda
What is chi kung fu panda Example of nation
Example of nation Country vs nation
Country vs nation Nation vs nation state
Nation vs nation state Wewujudan tegese
Wewujudan tegese Bugtong bugtong na may sagot
Bugtong bugtong na may sagot Fac med univ constantine 3
Fac med univ constantine 3 Faculté de médecine de constantine
Faculté de médecine de constantine Moodle univtln
Moodle univtln Fs univ umbb
Fs univ umbb Scolarité pharmacie nantes
Scolarité pharmacie nantes Lon-capa ohio university
Lon-capa ohio university Centre universitaire nour bachir el-bayadh
Centre universitaire nour bachir el-bayadh Http:fsi-st univ-boumerdes-dz
Http:fsi-st univ-boumerdes-dz Dr abou bekr
Dr abou bekr Univ constantine 3
Univ constantine 3 Iut valenciennes ent
Iut valenciennes ent Berechnung kalkulatorische zinsen
Berechnung kalkulatorische zinsen Prodoc univ nantes
Prodoc univ nantes State univ grant - sug ug
State univ grant - sug ug Sfa univ poitiers
Sfa univ poitiers Pharmacie univ batna 2
Pharmacie univ batna 2 Ent université tours
Ent université tours Univ of texas arlington
Univ of texas arlington Univ prof titel
Univ prof titel Umbb fs
Umbb fs (univ. caxias do sul) escolha a alternativa que completa
(univ. caxias do sul) escolha a alternativa que completa Scolarité médecine nantes
Scolarité médecine nantes Bier block
Bier block Alex cheng
Alex cheng Sophia cheng accident
Sophia cheng accident Cheng xiang zhai
Cheng xiang zhai Cheng
Cheng King cheng of zhou
King cheng of zhou Sega cheng
Sega cheng Routing table
Routing table Morrp
Morrp Cheng xiang zhai
Cheng xiang zhai Ck cheng ucsd
Ck cheng ucsd Peter chen er model
Peter chen er model Cộc cách tùng cheng
Cộc cách tùng cheng Cheng xiang zhai
Cheng xiang zhai Lou cheng
Lou cheng Cervical radiculopathy differential diagnosis
Cervical radiculopathy differential diagnosis Avery cheng
Avery cheng Cheng xiang zhai
Cheng xiang zhai Judy cheng hopkins
Judy cheng hopkins Chia liang cheng
Chia liang cheng Wei cheng lee
Wei cheng lee Cheng field and wave electromagnetics
Cheng field and wave electromagnetics Pauline cheng
Pauline cheng Cheng zhai
Cheng zhai Boey kim cheng
Boey kim cheng Dental alliteration
Dental alliteration Paradise by boey kim cheng analysis
Paradise by boey kim cheng analysis Vibrational mechanics
Vibrational mechanics Kiddonet games
Kiddonet games Cheng xiang zhai
Cheng xiang zhai Mktg 303
Mktg 303 Yizong cheng
Yizong cheng Antony cheng
Antony cheng Wayne cheng md
Wayne cheng md Cheng-few lee
Cheng-few lee Chung-kuan cheng
Chung-kuan cheng Cheng
Cheng Tiempo del discurso
Tiempo del discurso Res ipsa loquitur means
Res ipsa loquitur means Stp stratégia
Stp stratégia Tene rem verba sequentur
Tene rem verba sequentur In extrema res
In extrema res Lomo de aguja en estados unidos
Lomo de aguja en estados unidos Earth power lodge
Earth power lodge Definition of schizophrenia
Definition of schizophrenia Gh
Gh Res incorporalis
Res incorporalis