20 3 Nucleophilic Substitution in Acyl Chlorides General

20. 3 Nucleophilic Substitution in Acyl Chlorides
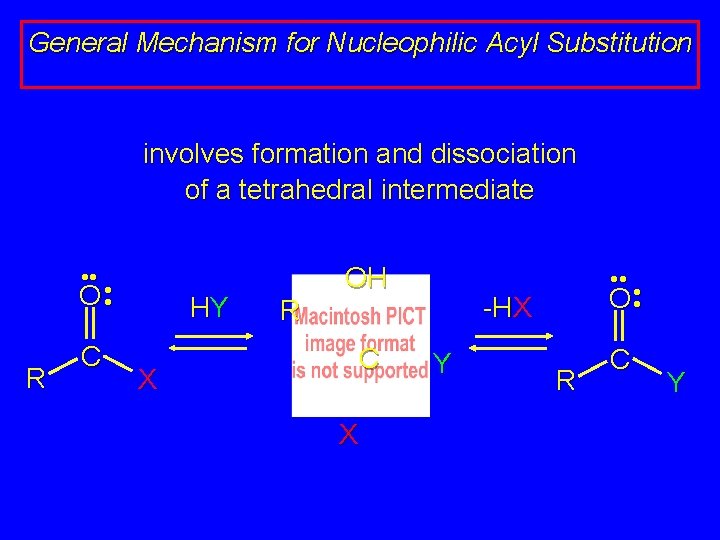
General Mechanism for Nucleophilic Acyl Substitution involves formation and dissociation of a tetrahedral intermediate • • O • • R C HY OH -HX R C X X • • O • • Y R C Y
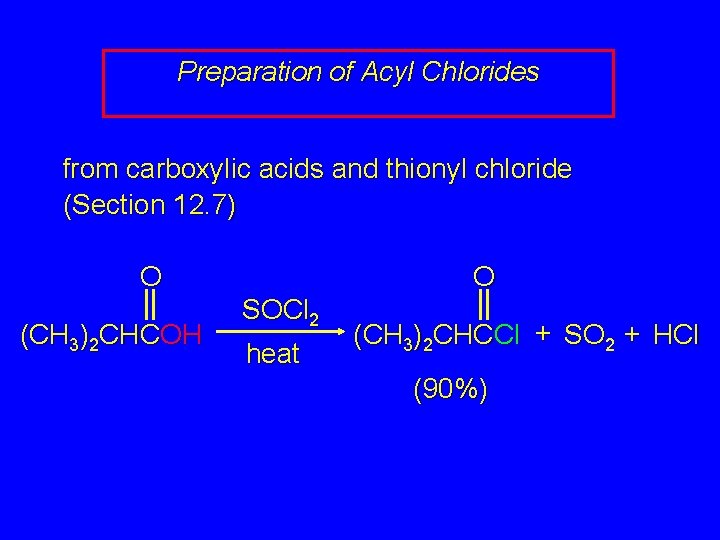
Preparation of Acyl Chlorides from carboxylic acids and thionyl chloride (Section 12. 7) O (CH 3)2 CHCOH O SOCl 2 heat (CH 3)2 CHCCl + SO 2 + HCl (90%)
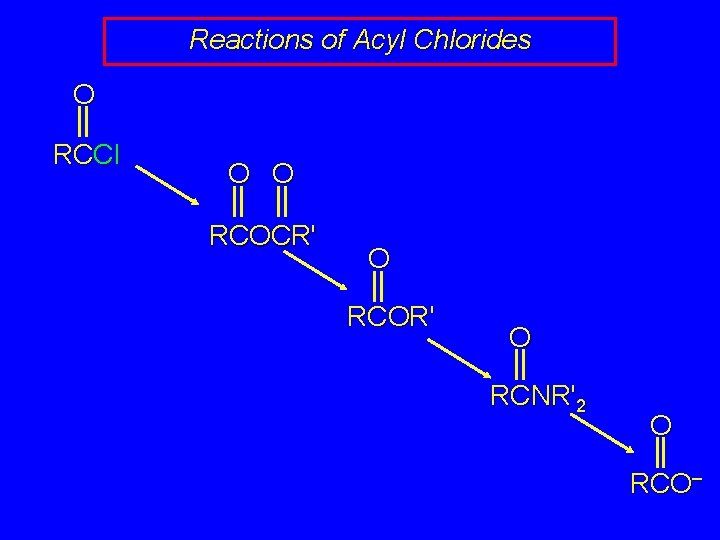
Reactions of Acyl Chlorides O RCCl O O RCOCR' O RCOR' O RCNR'2 O RCO–
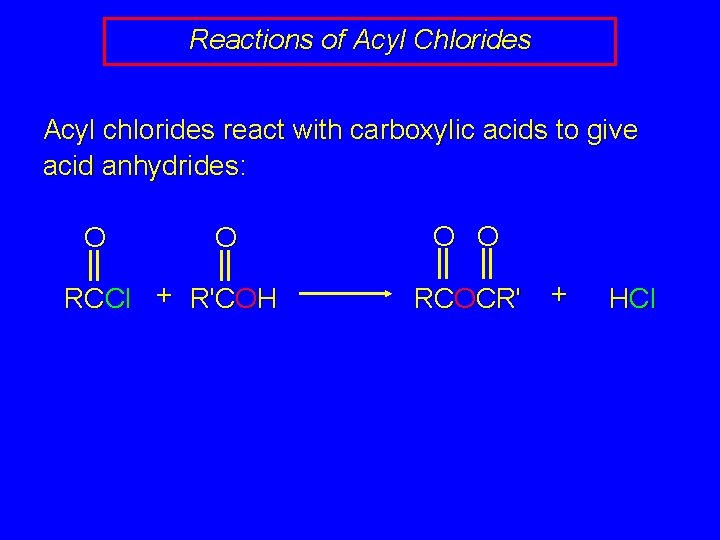
Reactions of Acyl Chlorides Acyl chlorides react with carboxylic acids to give acid anhydrides: O O RCCl + R'COH O O RCOCR' + HCl
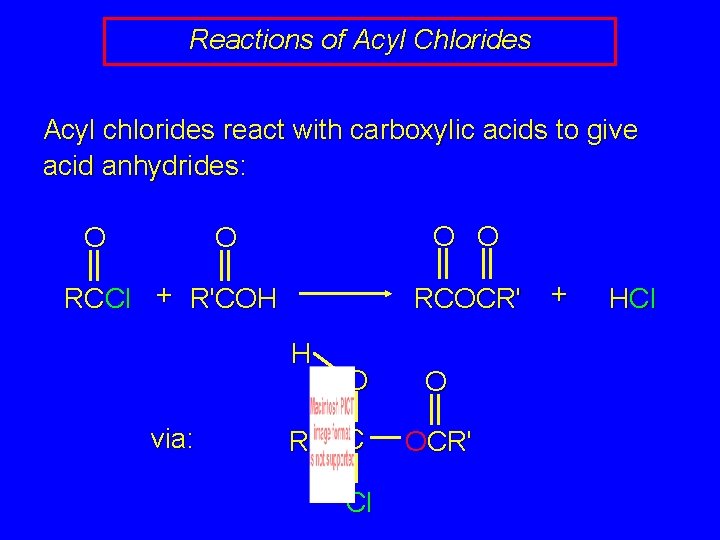
Reactions of Acyl Chlorides Acyl chlorides react with carboxylic acids to give acid anhydrides: O O RCCl + R'COH RCOCR' H via: R O O C OCR' Cl + HCl
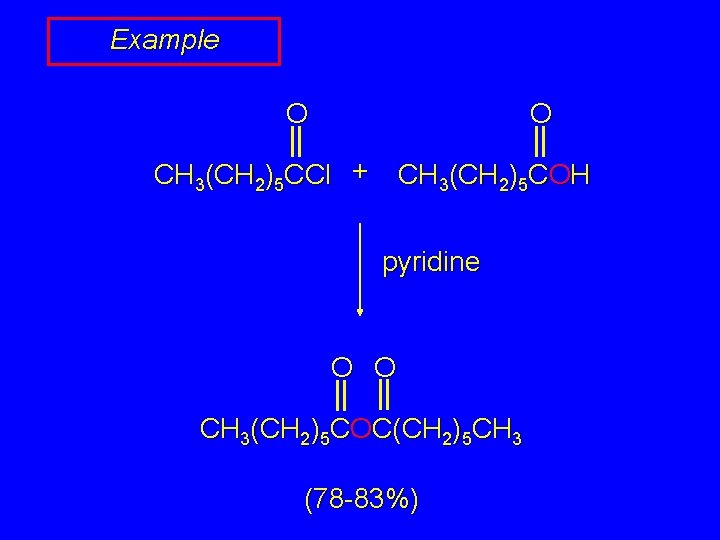
Example O O CH 3(CH 2)5 CCl + CH 3(CH 2)5 COH pyridine O O CH 3(CH 2)5 COC(CH 2)5 CH 3 (78 -83%)
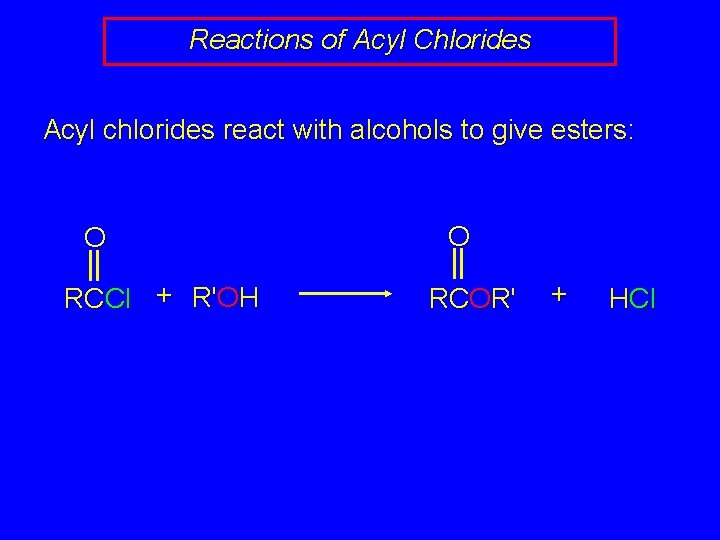
Reactions of Acyl Chlorides Acyl chlorides react with alcohols to give esters: O RCCl + R'OH O RCOR' + HCl
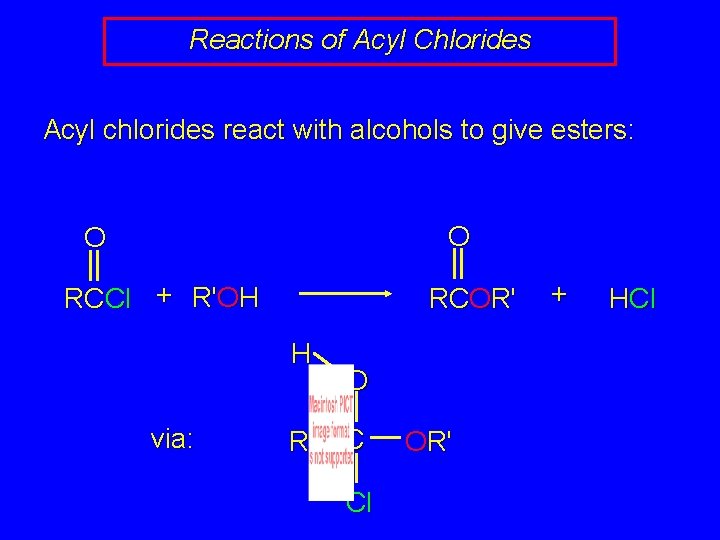
Reactions of Acyl Chlorides Acyl chlorides react with alcohols to give esters: O O RCCl + R'OH RCOR' H via: R O C Cl OR' + HCl
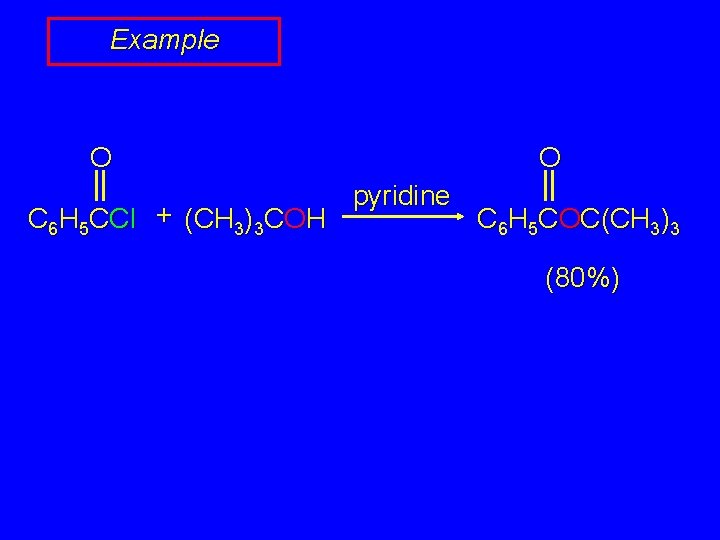
Example O O C 6 H 5 CCl + (CH 3)3 COH pyridine C 6 H 5 COC(CH 3)3 (80%)
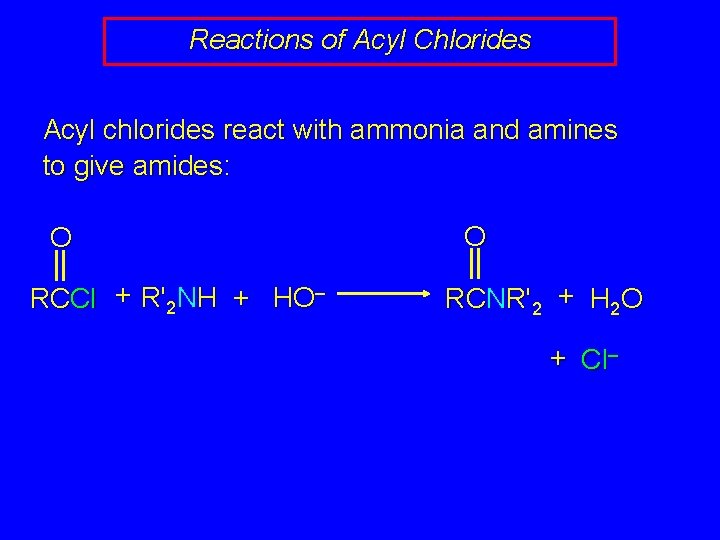
Reactions of Acyl Chlorides Acyl chlorides react with ammonia and amines to give amides: O RCCl + R'2 NH + HO– O RCNR'2 + H 2 O + Cl–
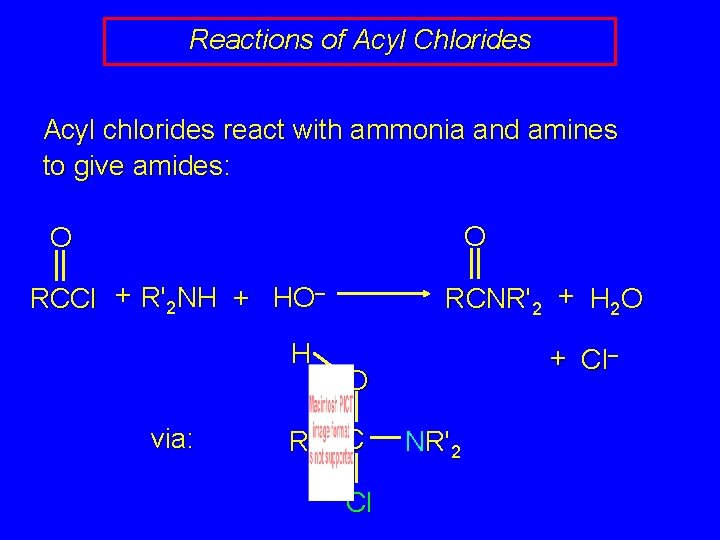
Reactions of Acyl Chlorides Acyl chlorides react with ammonia and amines to give amides: O O RCCl + R'2 NH + HO– H via: R RCNR'2 + H 2 O + Cl– O C Cl NR'2
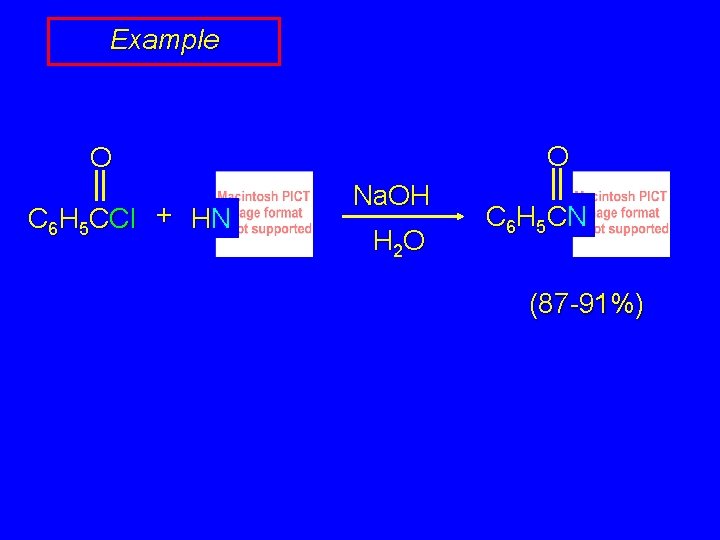
Example O O C 6 H 5 CCl + HN Na. OH H 2 O C 6 H 5 C N (87 -91%)
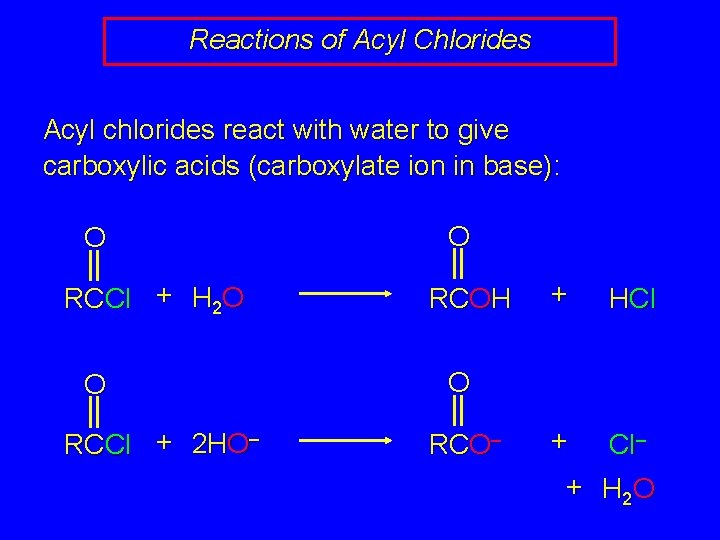
Reactions of Acyl Chlorides Acyl chlorides react with water to give carboxylic acids (carboxylate ion in base): O RCCl + H 2 O O RCCl + 2 HO– O RCOH + HCl + Cl– O RCO– + H 2 O
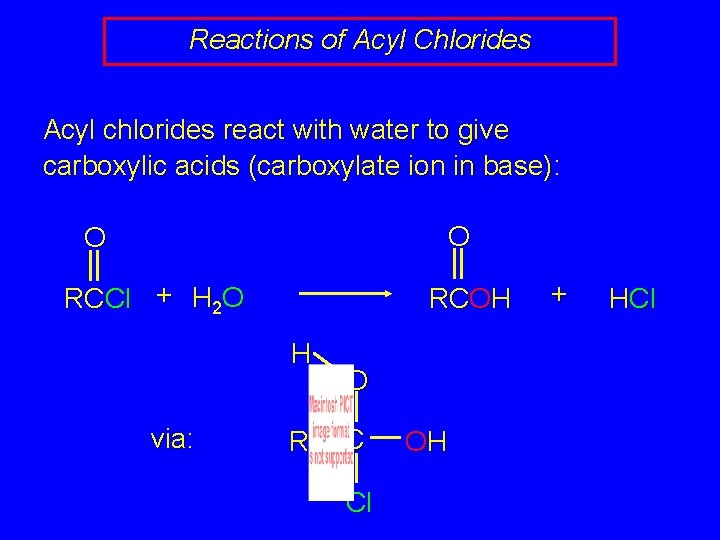
Reactions of Acyl Chlorides Acyl chlorides react with water to give carboxylic acids (carboxylate ion in base): O O RCCl + H 2 O RCOH H via: R O C Cl OH + HCl
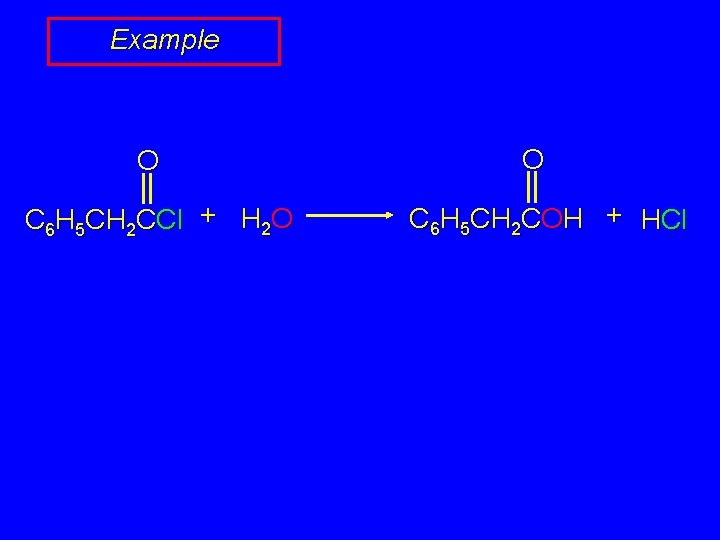
Example O C 6 H 5 CH 2 CCl + H 2 O O C 6 H 5 CH 2 COH + HCl
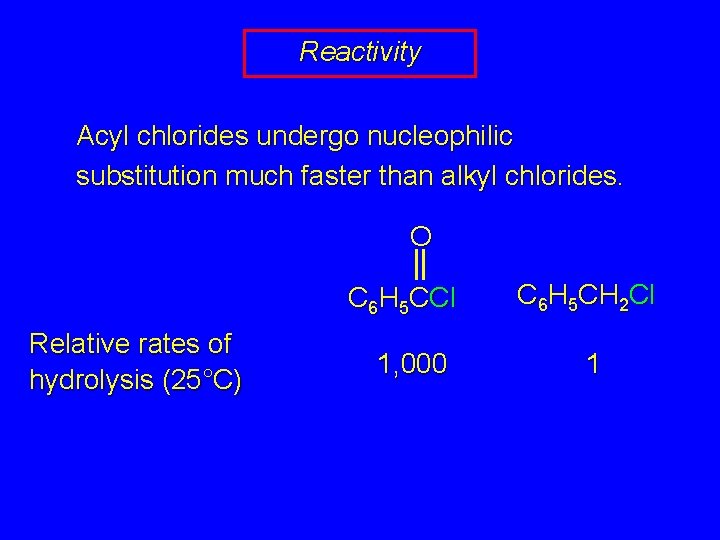
Reactivity Acyl chlorides undergo nucleophilic substitution much faster than alkyl chlorides. O C 6 H 5 CCl Relative rates of hydrolysis (25°C) 1, 000 C 6 H 5 CH 2 Cl 1

20. 4 Preparation of Carboxylic Acid Anhydrides can be prepared from acyl chlorides as described in Table 20. 2
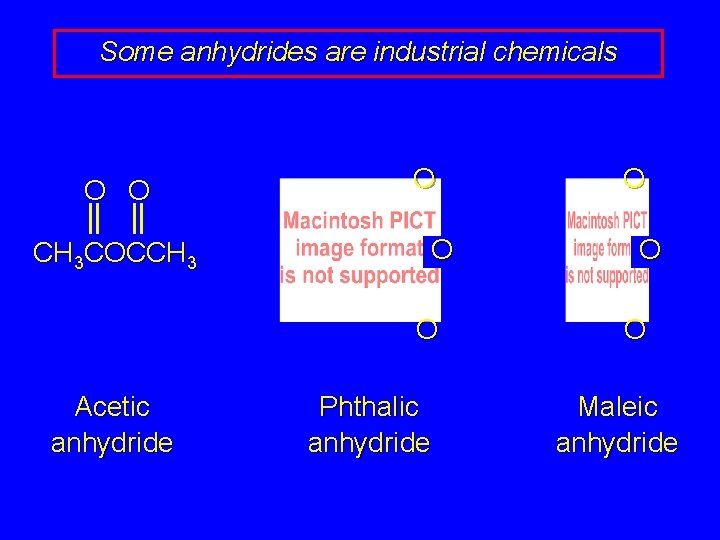
Some anhydrides are industrial chemicals O O CH 3 COCCH 3 O O O Acetic anhydride Phthalic anhydride O O O Maleic anhydride
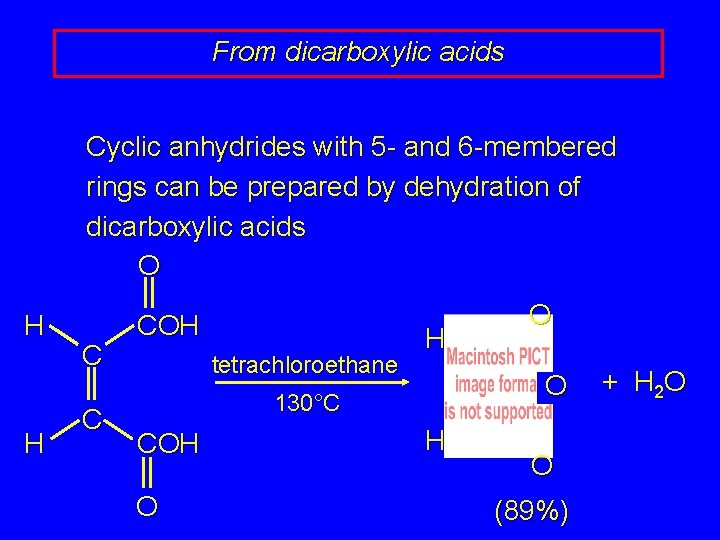
From dicarboxylic acids Cyclic anhydrides with 5 - and 6 -membered rings can be prepared by dehydration of dicarboxylic acids O H H C C COH tetrachloroethane H O 130°C COH O O H O (89%) + H 2 O

20. 5 Reactions of Carboxylic Acid Anhydrides Table 20. 3
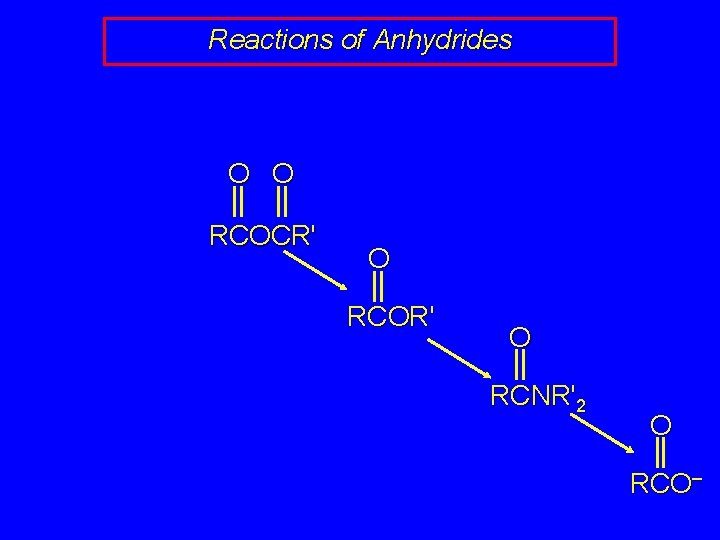
Reactions of Anhydrides O O RCOCR' O RCOR' O RCNR'2 O RCO–
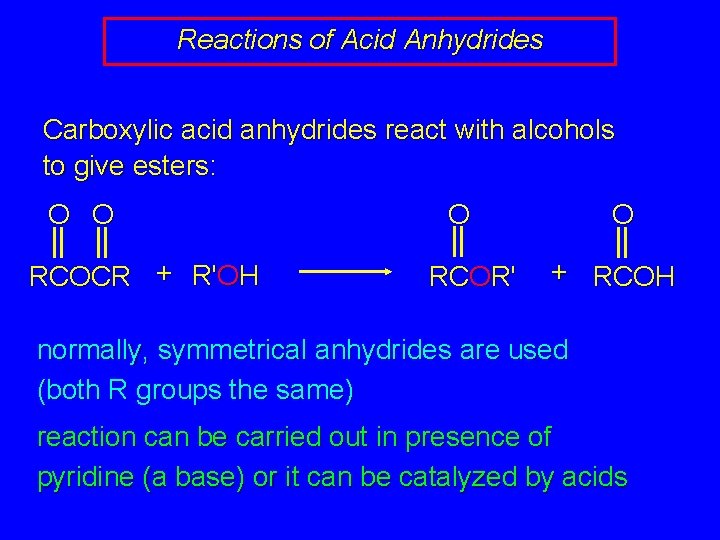
Reactions of Acid Anhydrides Carboxylic acid anhydrides react with alcohols to give esters: O O RCOCR + R'OH O RCOR' O + RCOH normally, symmetrical anhydrides are used (both R groups the same) reaction can be carried out in presence of pyridine (a base) or it can be catalyzed by acids
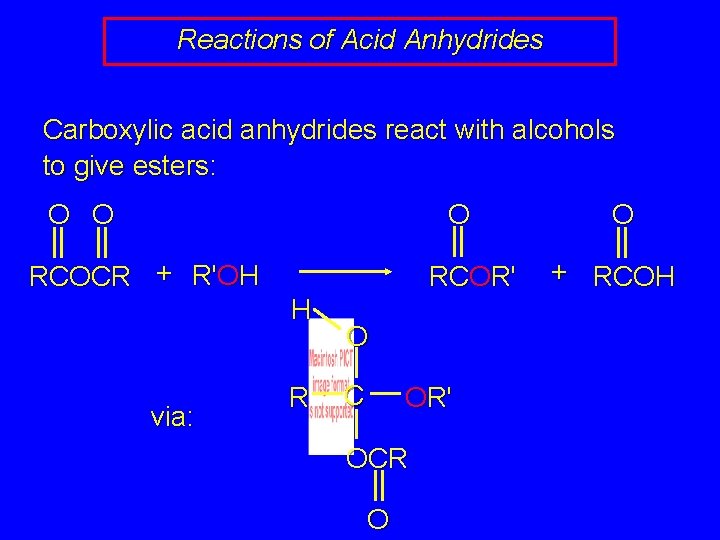
Reactions of Acid Anhydrides Carboxylic acid anhydrides react with alcohols to give esters: O O O RCOCR + R'OH RCOR' H via: R O C OR' OCR O O + RCOH
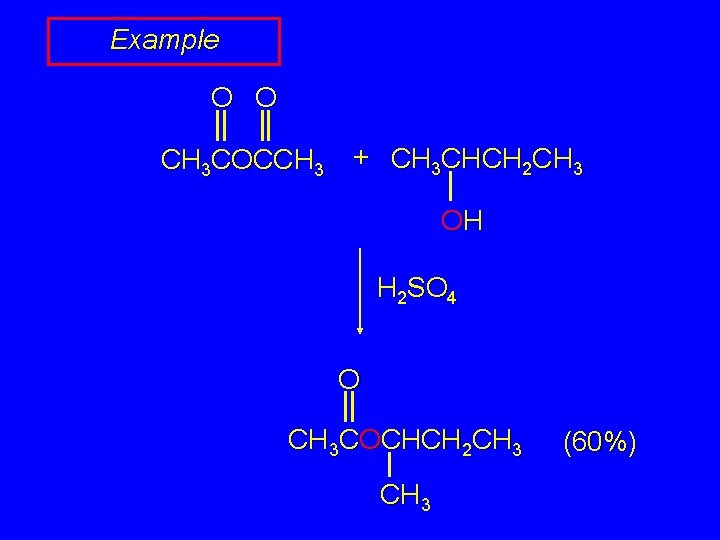
Example O O CH 3 COCCH 3 + CH 3 CHCH 2 CH 3 OH H 2 SO 4 O CH 3 COCHCH 2 CH 3 (60%)
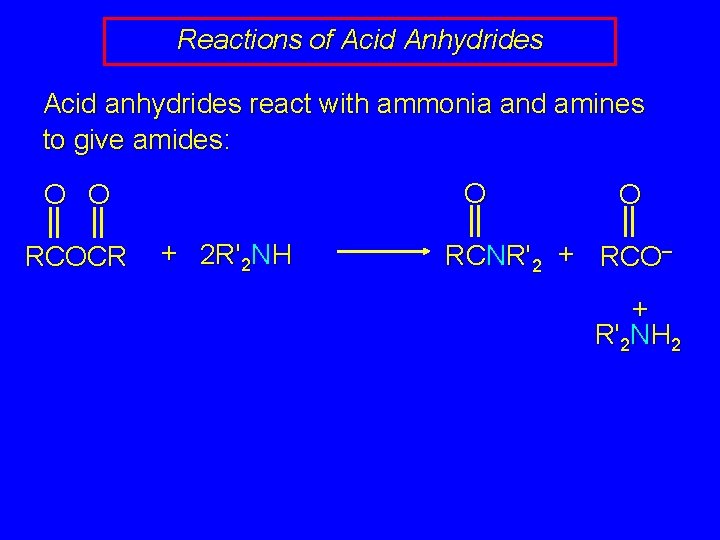
Reactions of Acid Anhydrides Acid anhydrides react with ammonia and amines to give amides: O O O RCOCR + 2 R'2 NH O RCNR'2 + RCO– + R'2 NH 2
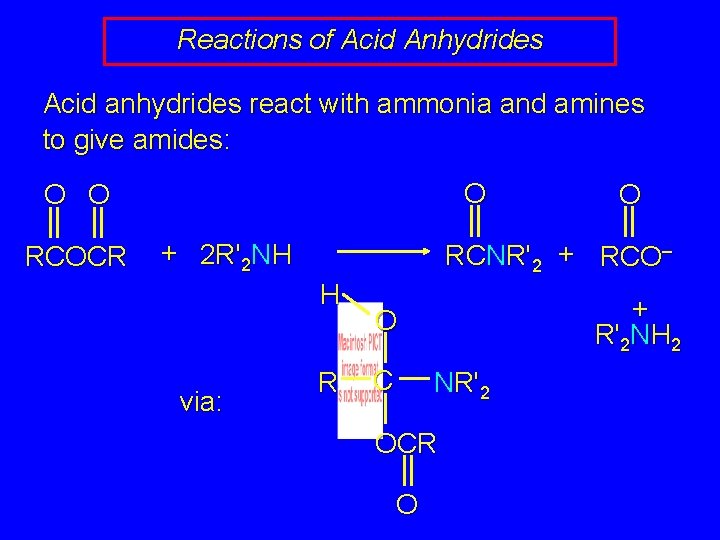
Reactions of Acid Anhydrides Acid anhydrides react with ammonia and amines to give amides: O O O RCOCR + 2 R'2 NH RCNR'2 + RCO– H via: O R + R'2 NH 2 O C NR'2 OCR O
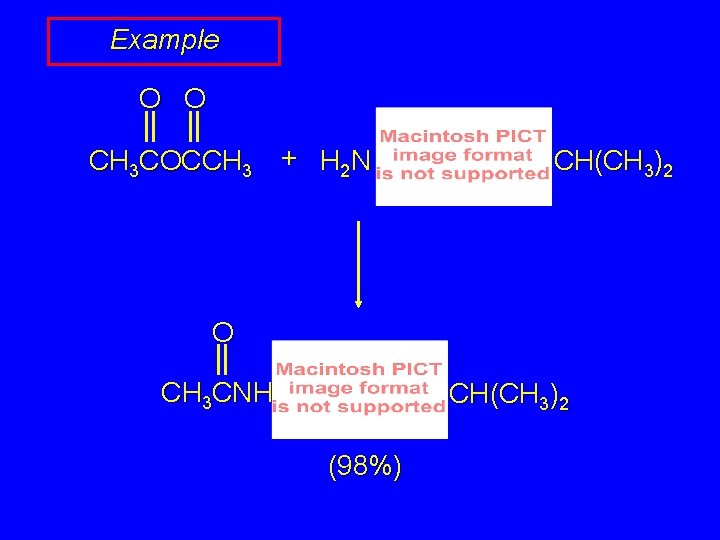
Example O O CH 3 COCCH 3 + H 2 N CH(CH 3)2 O CH 3 CNH CH(CH 3)2 (98%)
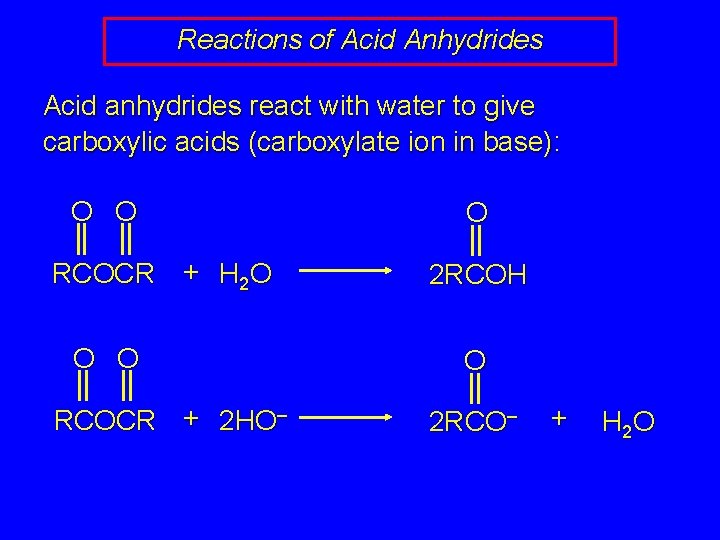
Reactions of Acid Anhydrides Acid anhydrides react with water to give carboxylic acids (carboxylate ion in base): O O RCOCR + H 2 O O O RCOCR + 2 HO– O 2 RCOH O 2 RCO– + H 2 O
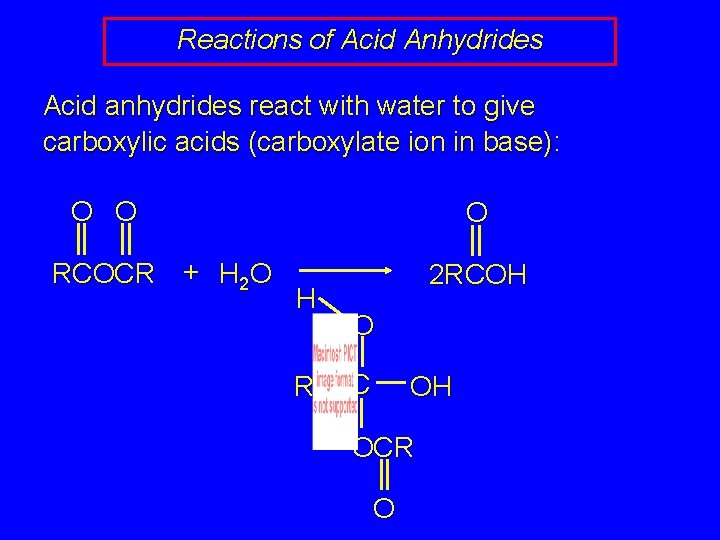
Reactions of Acid Anhydrides Acid anhydrides react with water to give carboxylic acids (carboxylate ion in base): O O RCOCR + H 2 O O H R 2 RCOH O C OH OCR O
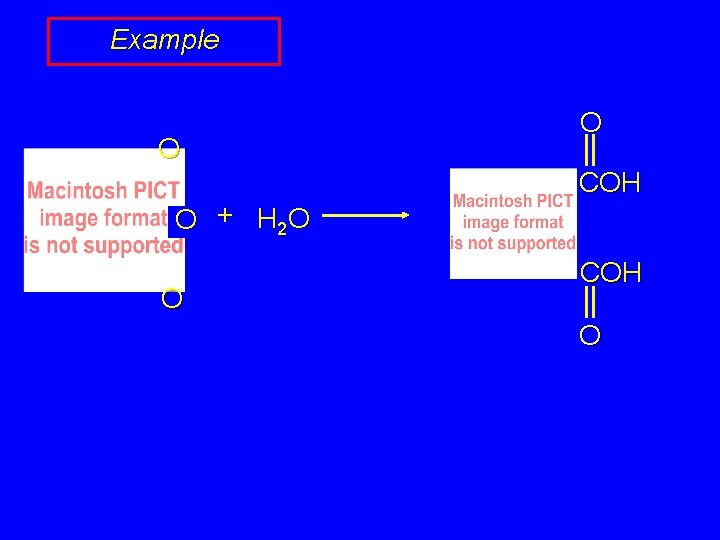
Example O O + H 2 O O O COH O
- Slides: 31