20 13 Preparation of Amides Preparation of Amides











- Slides: 50

20. 13 Preparation of Amides

Preparation of Amides are prepared from amines by acylation with: acyl chlorides (Table 20. 2) anhydrides (Table 20. 3) esters (Table 20. 6)

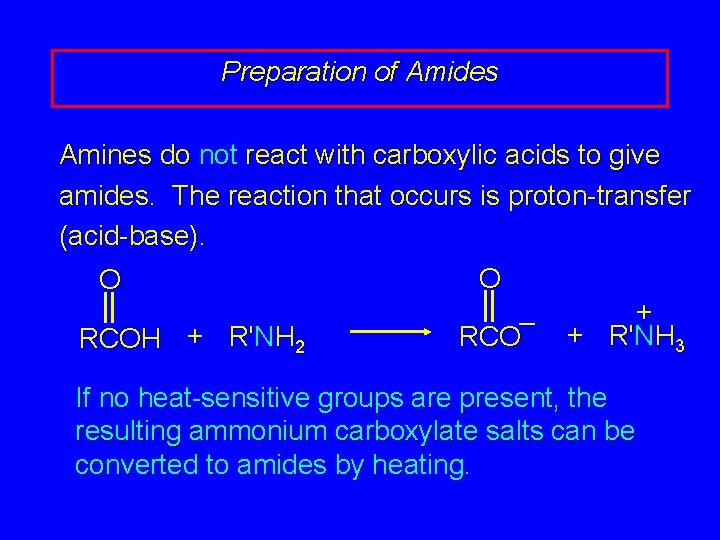
Preparation of Amides Amines do not react with carboxylic acids to give amides. The reaction that occurs is proton-transfer (acid-base). O O + – + R'NH 3 RCOH + R'NH 2 If no heat-sensitive groups are present, the resulting ammonium carboxylate salts can be converted to amides by heating.

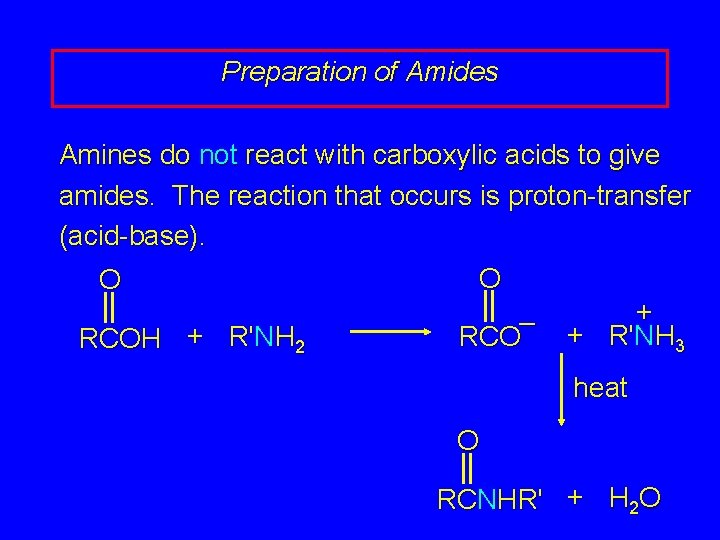
Preparation of Amides Amines do not react with carboxylic acids to give amides. The reaction that occurs is proton-transfer (acid-base). O O + – + R'NH 3 RCOH + R'NH 2 heat O RCNHR' + H 2 O

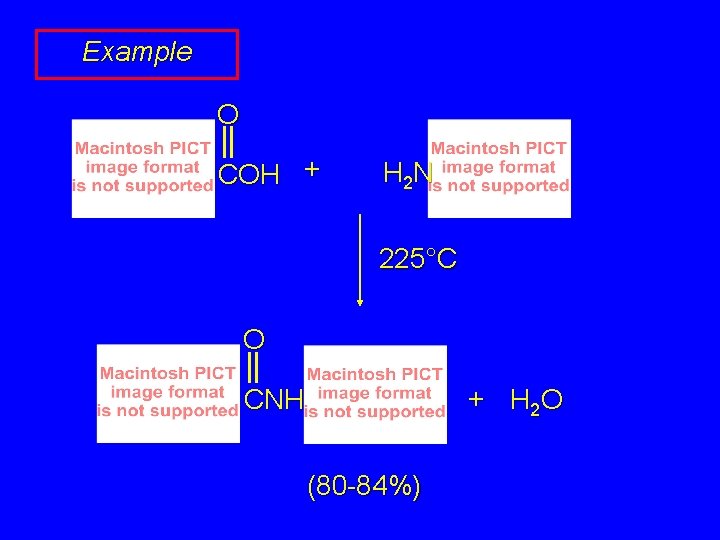
Example O COH + H 2 N 225°C O + H 2 O CNH (80 -84%)

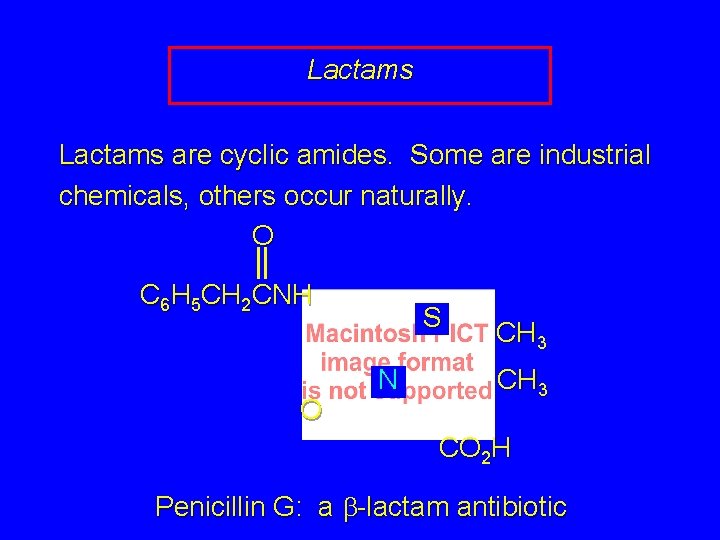
20. 14 Lactams

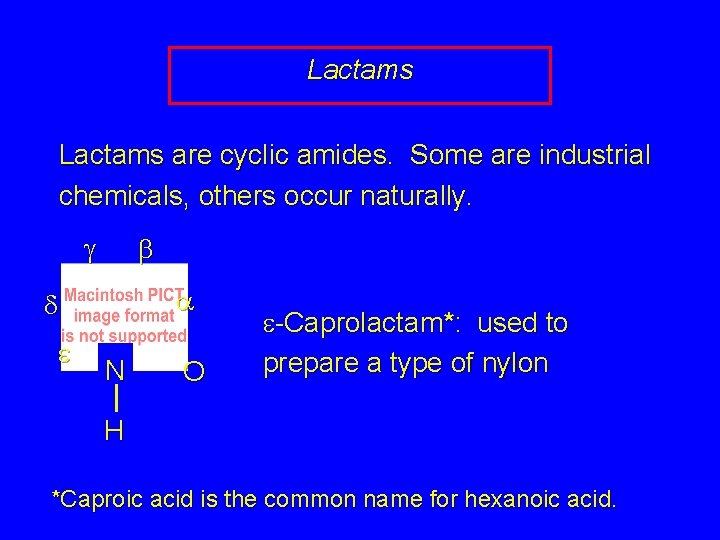
Lactams are cyclic amides. Some are industrial chemicals, others occur naturally. g b a d e N O e-Caprolactam*: used to prepare a type of nylon H *Caproic acid is the common name for hexanoic acid.

Lactams are cyclic amides. Some are industrial chemicals, others occur naturally. O C 6 H 5 CH 2 CNH a O b S N CH 3 CO 2 H Penicillin G: a b-lactam antibiotic

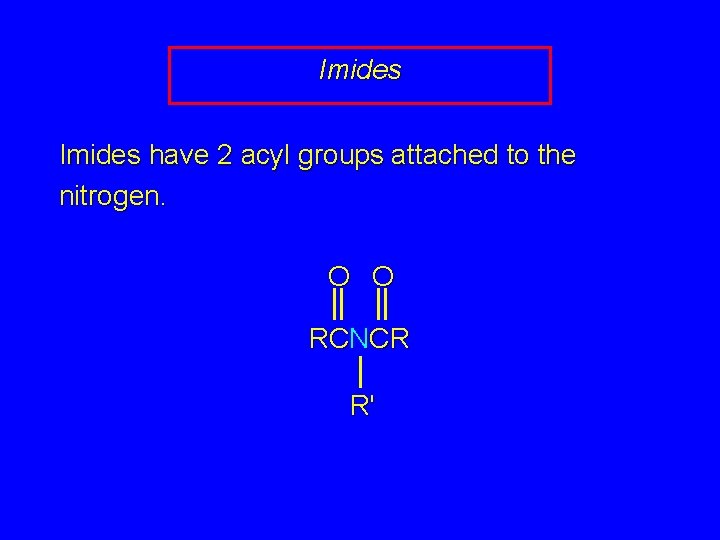
20. 15 Imides

Imides have 2 acyl groups attached to the nitrogen. O O RCNCR R'

Imides The most common examples are cyclic imides. O NH O Succinimide O NH O Phthalimide

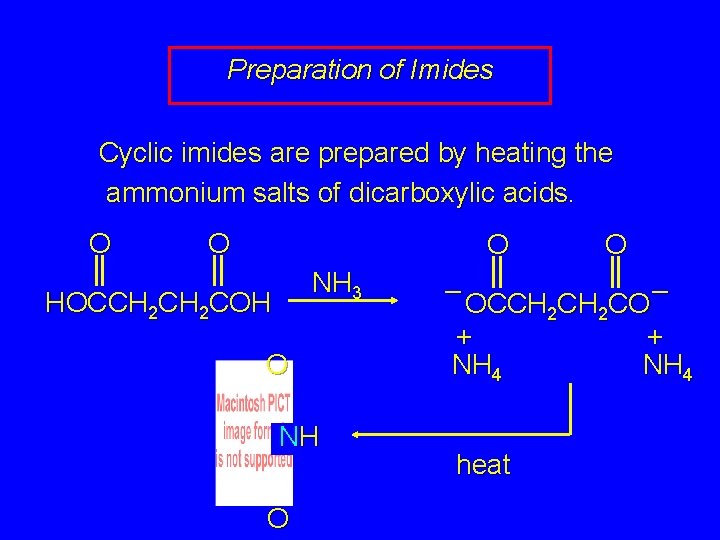
Preparation of Imides Cyclic imides are prepared by heating the ammonium salts of dicarboxylic acids. O O O NH 3 HOCCH 2 COH O NH O O – – OCCH 2 CO + + NH 4 heat

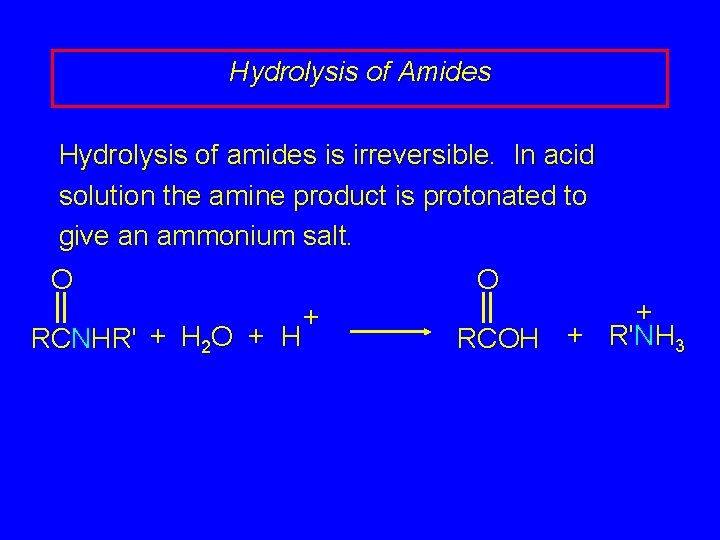
20. 16 Hydrolysis of Amides

Hydrolysis of Amides Hydrolysis of amides is irreversible. In acid solution the amine product is protonated to give an ammonium salt. O RCNHR' + H 2 O + H O + + RCOH + R'NH 3

Hydrolysis of Amides In basic solution the carboxylic acid product is deprotonated to give a carboxylate ion. O RCNHR' O – + HO – RCO + R'NH 2

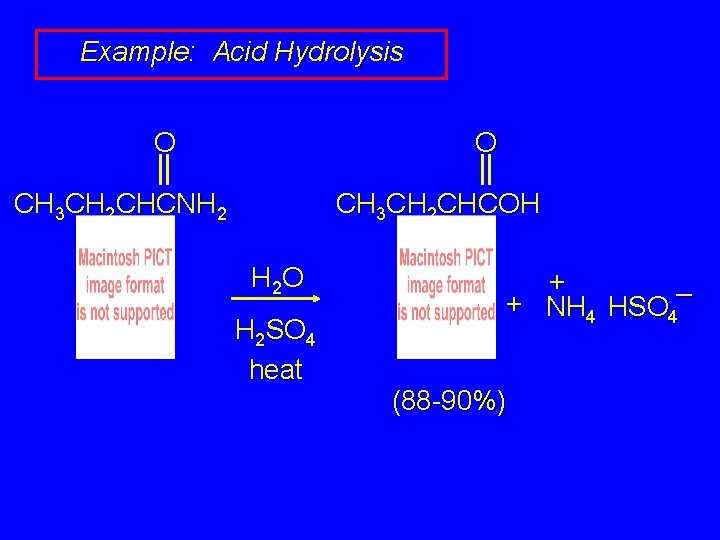
Example: Acid Hydrolysis O O CH 3 CH 2 CHCNH 2 CH 3 CH 2 CHCOH H 2 O H 2 SO 4 heat + + NH 4 HSO 4– (88 -90%)

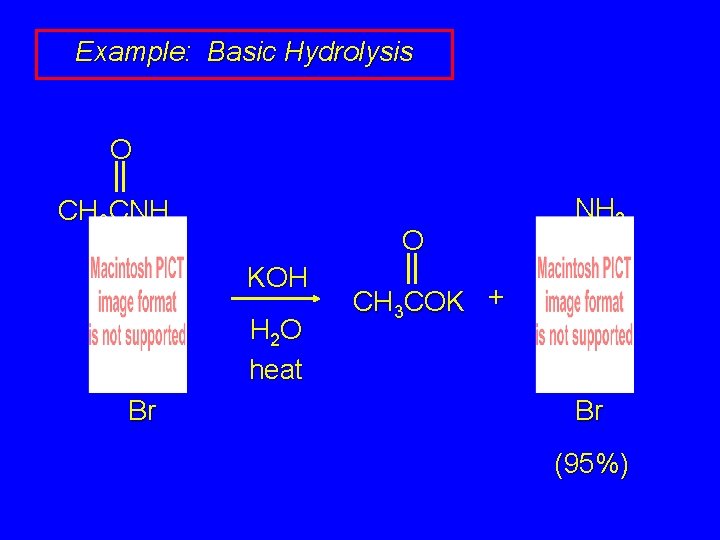
Example: Basic Hydrolysis O CH 3 CNH O KOH H 2 O heat Br NH 2 CH 3 COK + Br (95%)

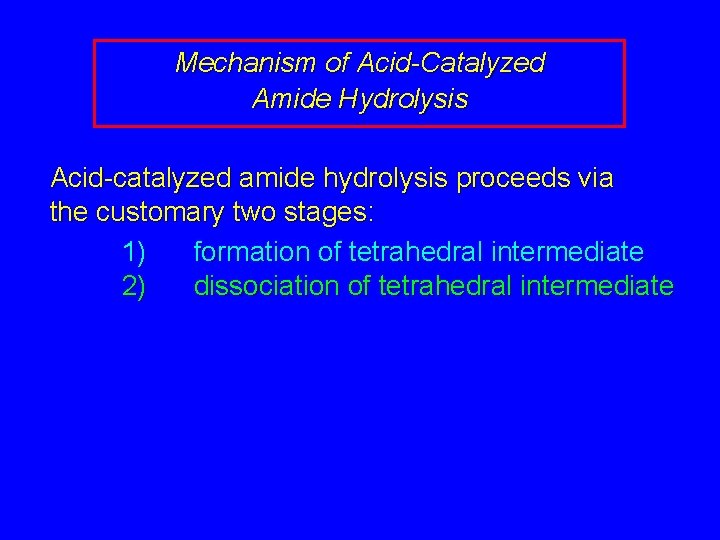
Mechanism of Acid-Catalyzed Amide Hydrolysis Acid-catalyzed amide hydrolysis proceeds via the customary two stages: 1) formation of tetrahedral intermediate 2) dissociation of tetrahedral intermediate

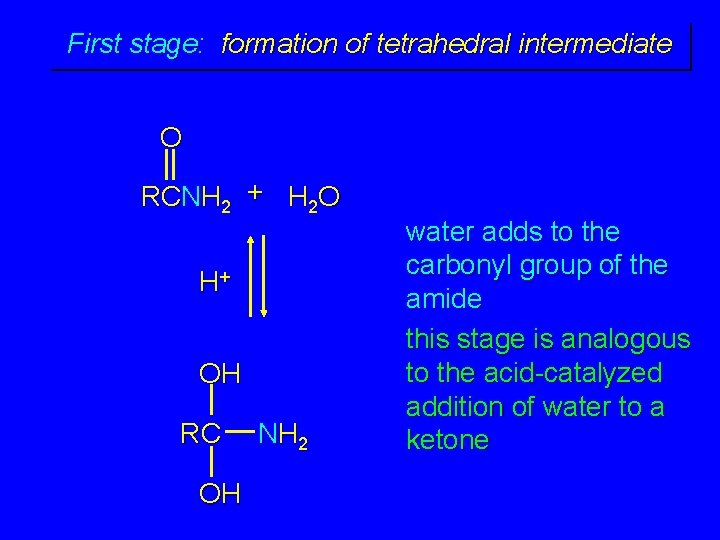
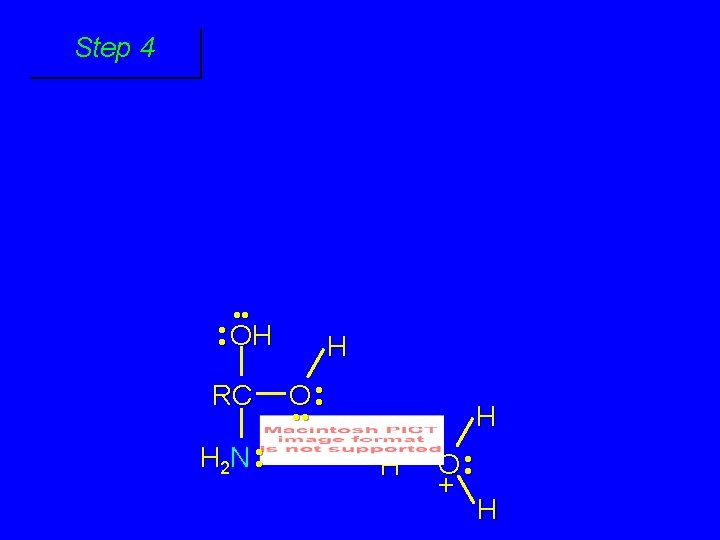
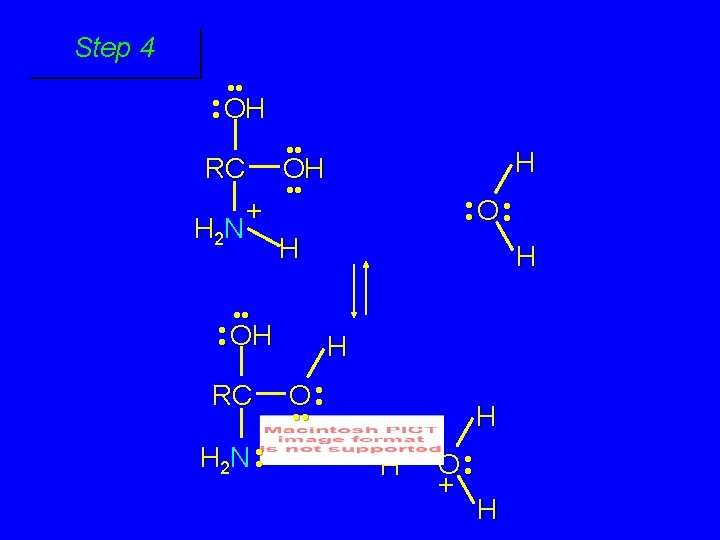
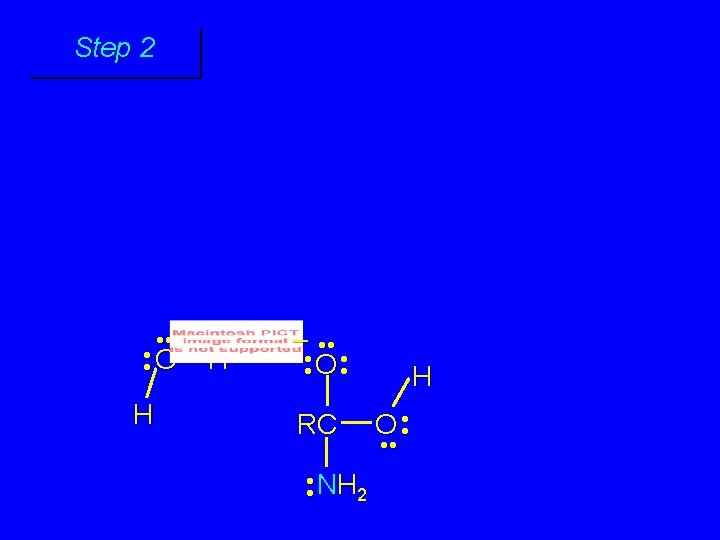
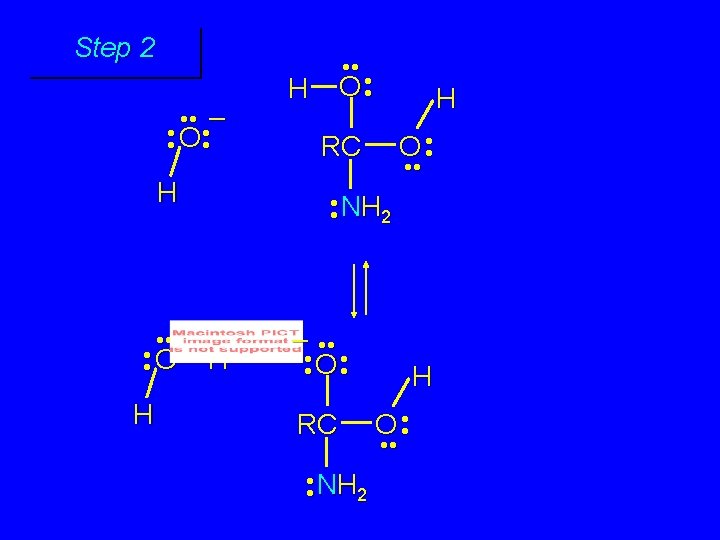
First stage: formation of tetrahedral intermediate O RCNH 2 + H 2 O H+ OH RC OH NH 2 water adds to the carbonyl group of the amide this stage is analogous to the acid-catalyzed addition of water to a ketone

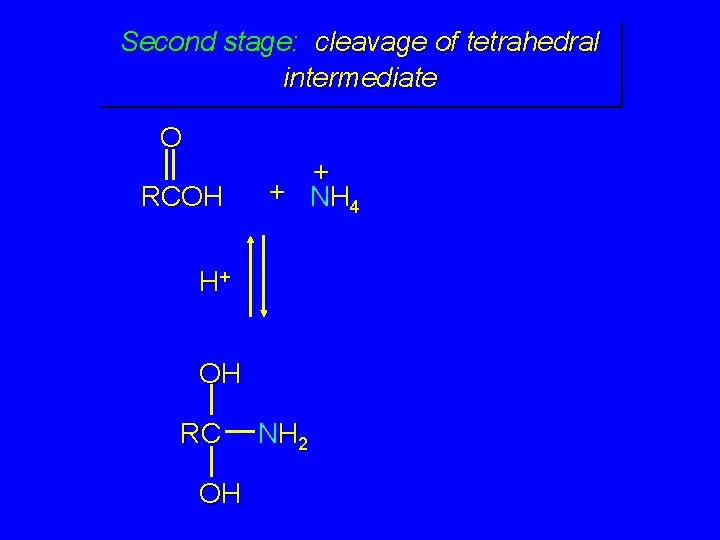
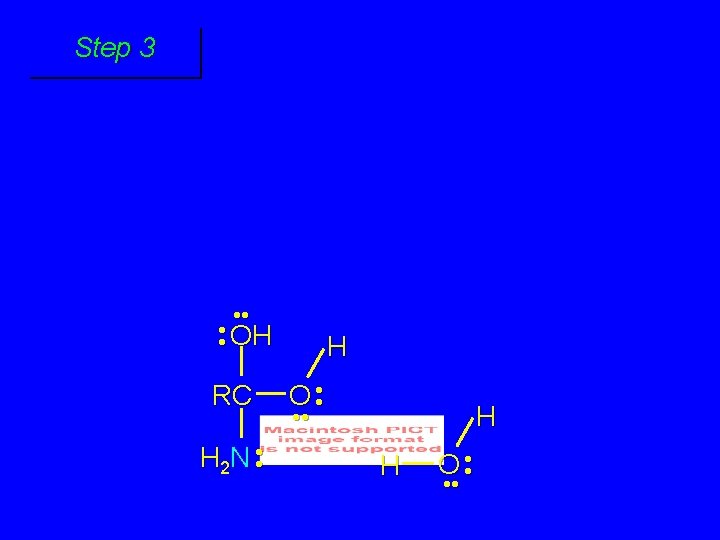
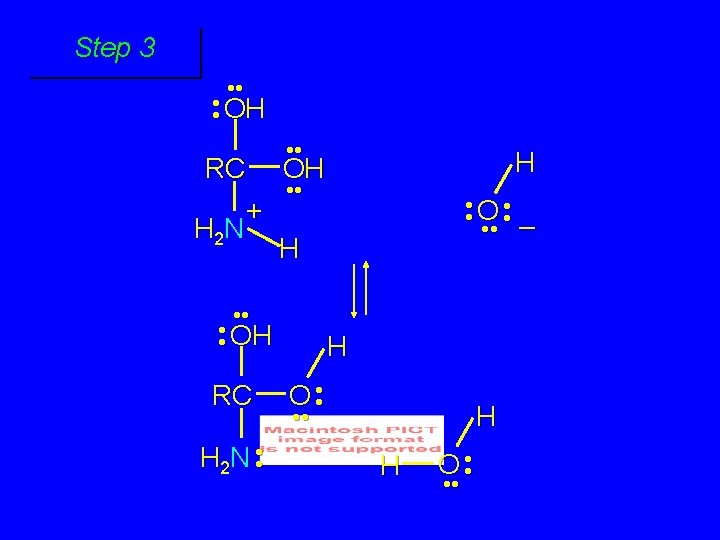
Second stage: cleavage of tetrahedral intermediate O RCOH + + NH 4 H+ OH RC OH NH 2

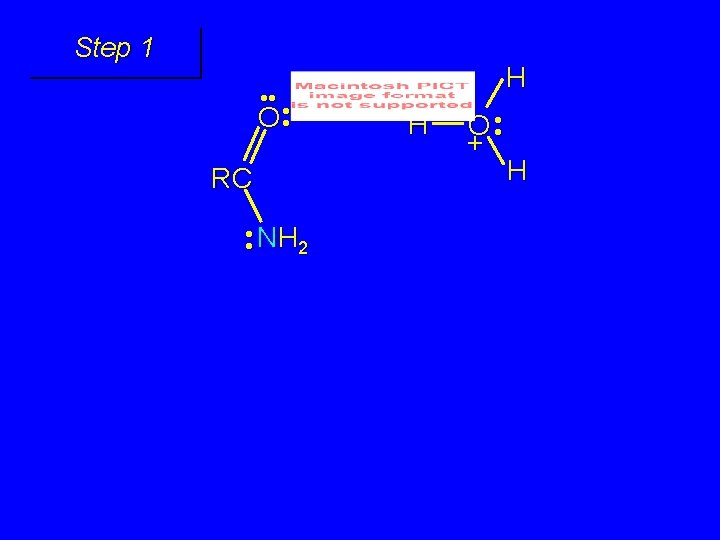
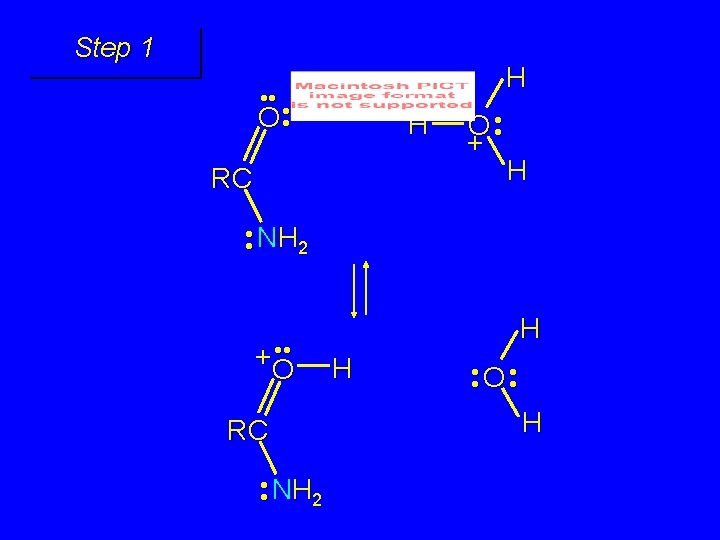
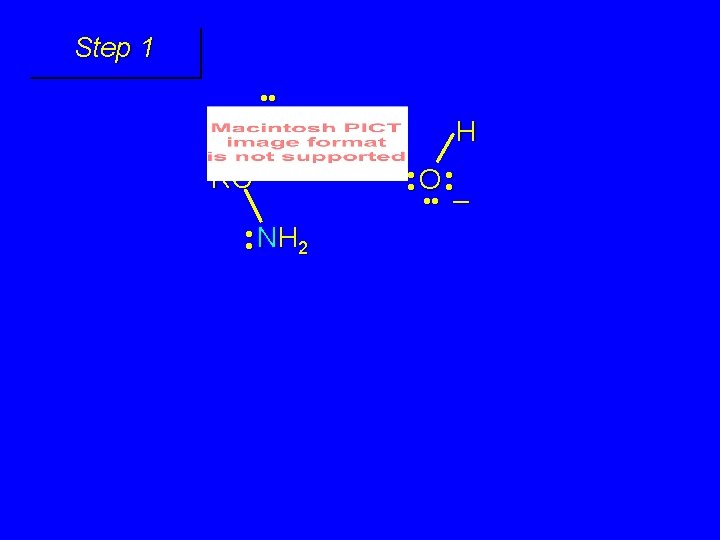
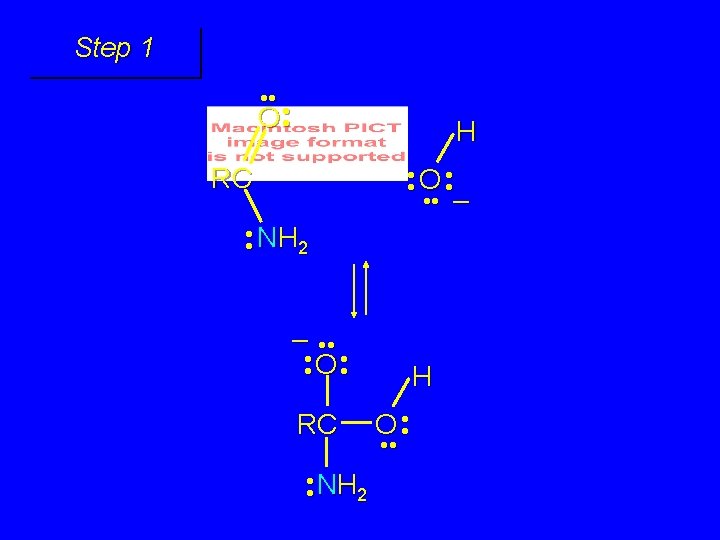
Mechanism of formation of tetrahedral intermediate



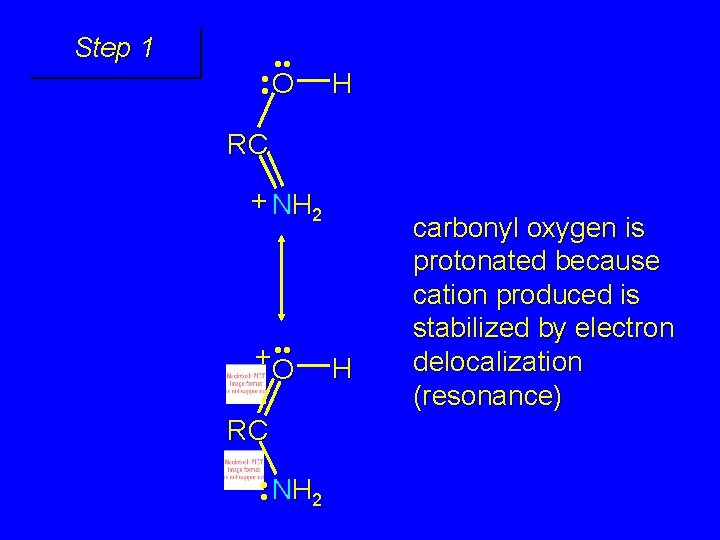
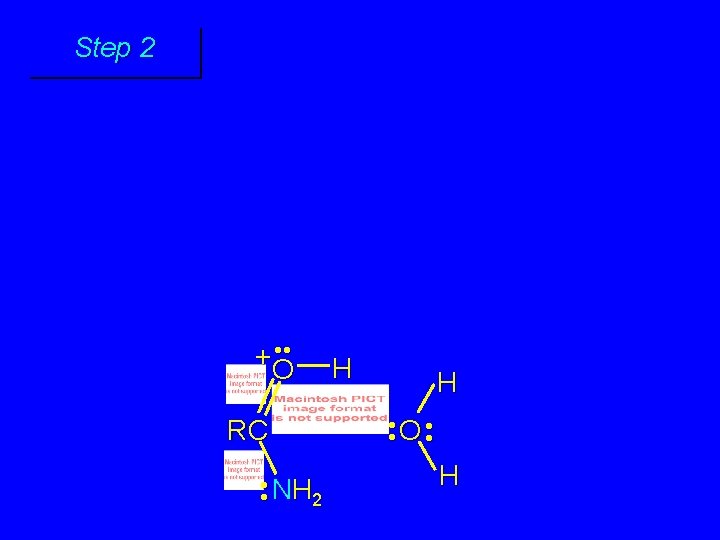
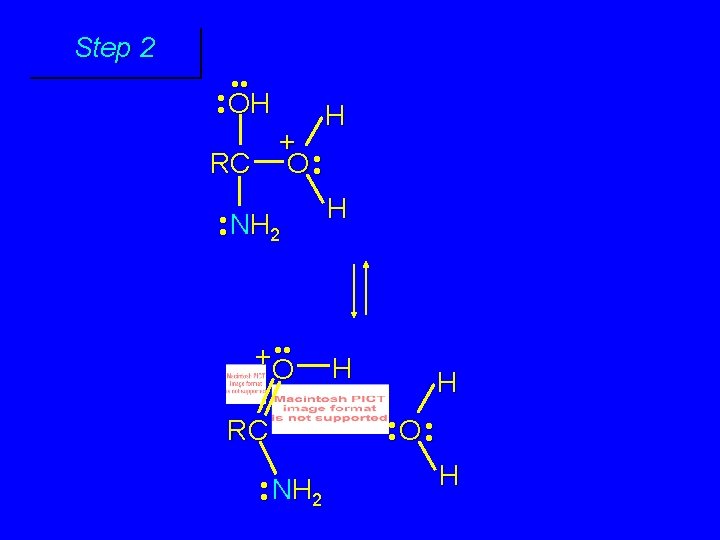
Step 1 • • O H RC + NH 2 • • +O RC • • NH 2 H carbonyl oxygen is protonated because cation produced is stabilized by electron delocalization (resonance)





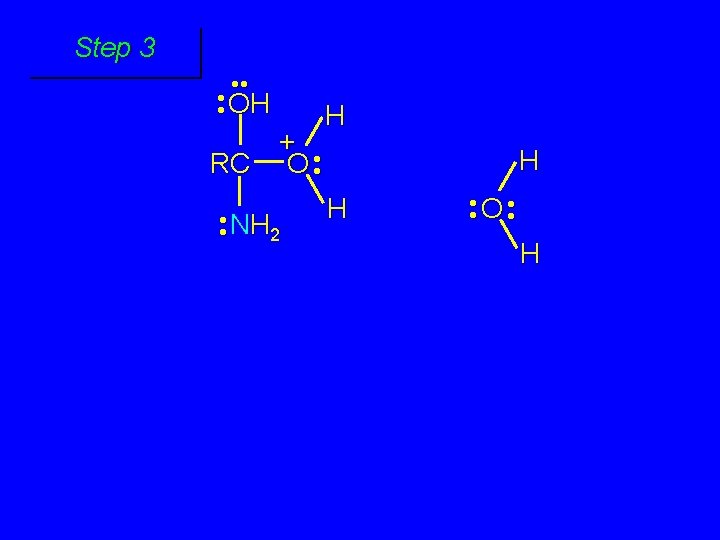
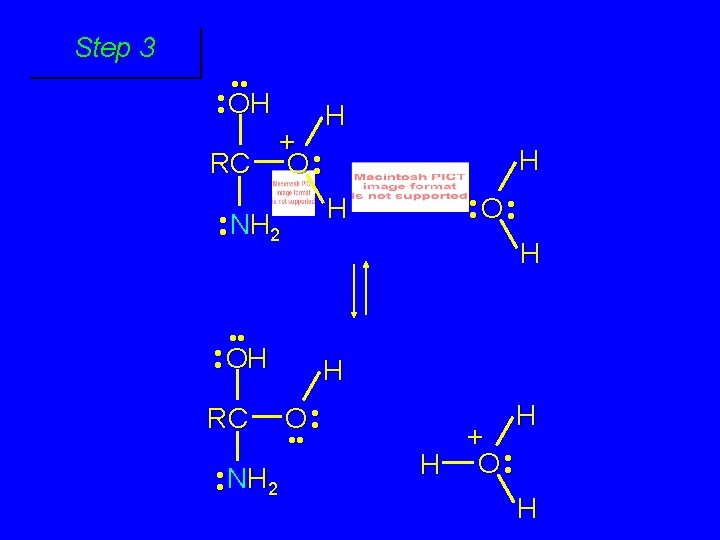
Cleavage of tetrahedral intermediate








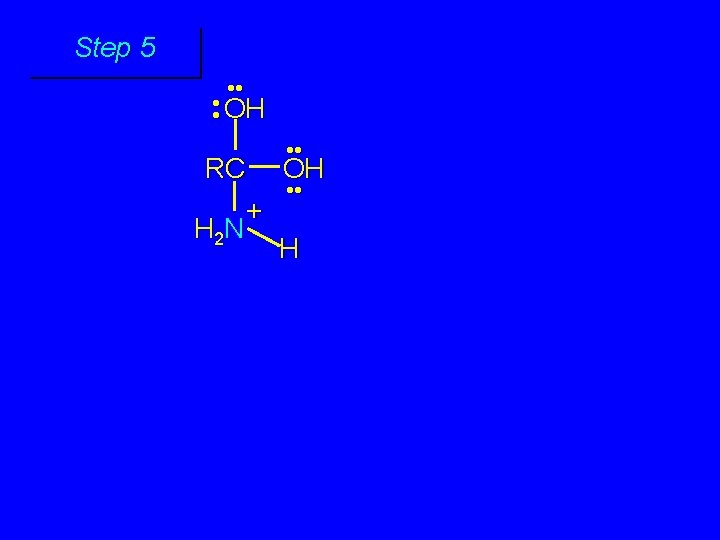
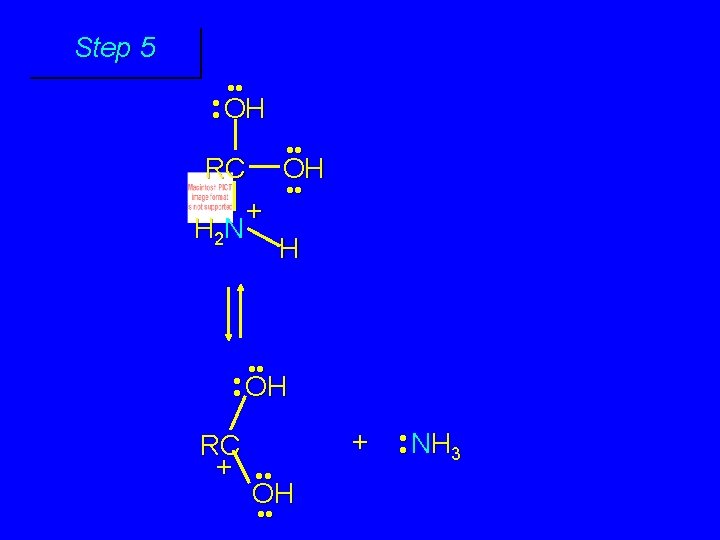
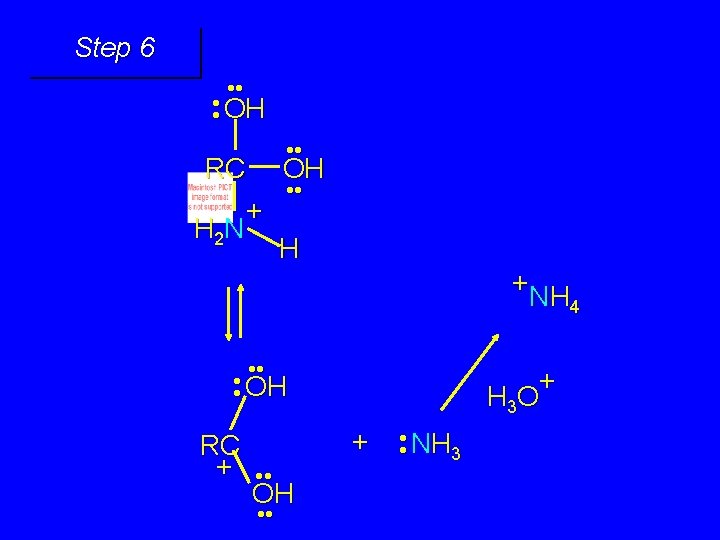
Mechanism of Amide Hydrolysis in Base Involves two stages: 1) formation of tetrahedral intermediate 2) dissociation of tetrahedral intermediate

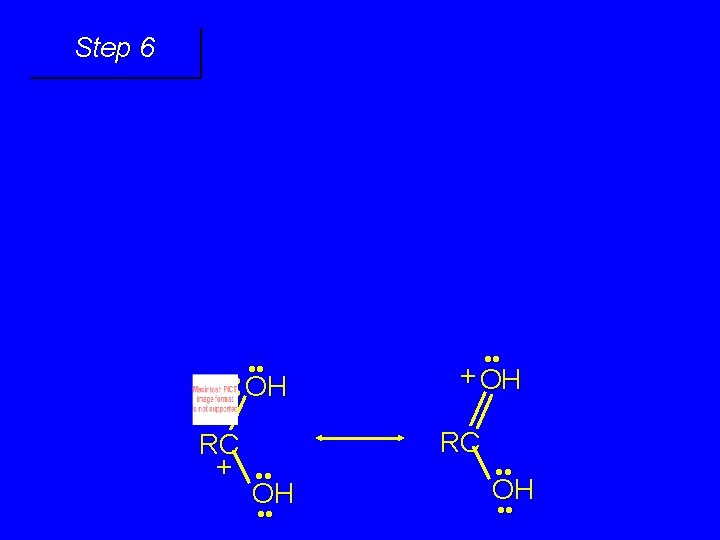
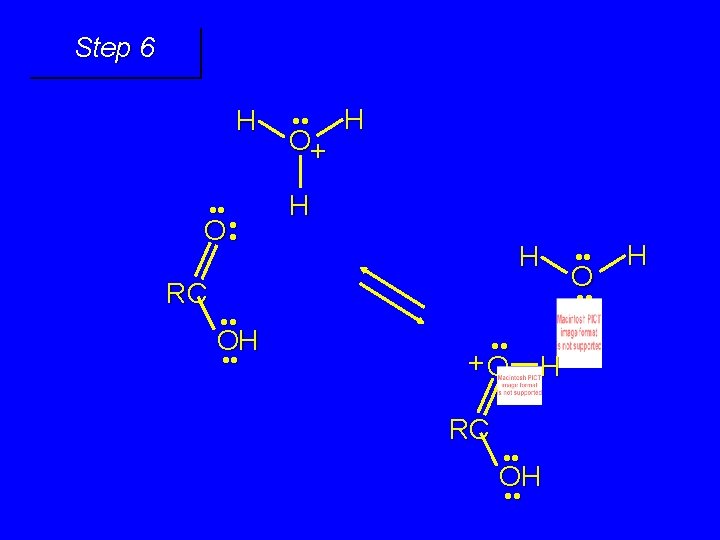
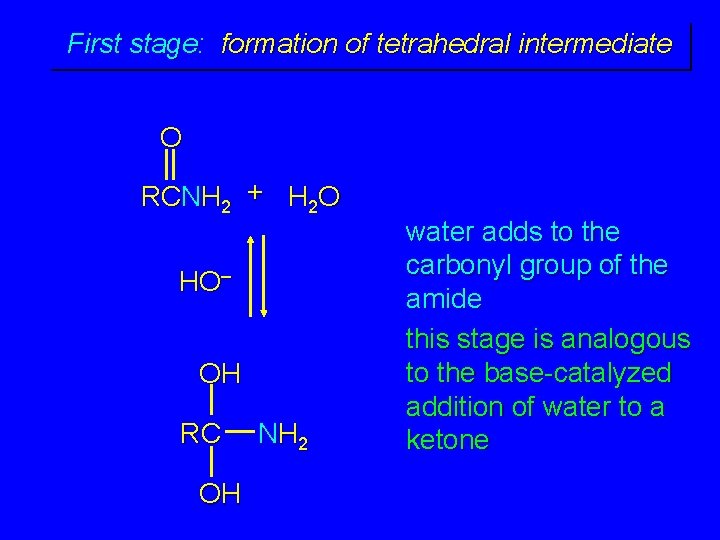
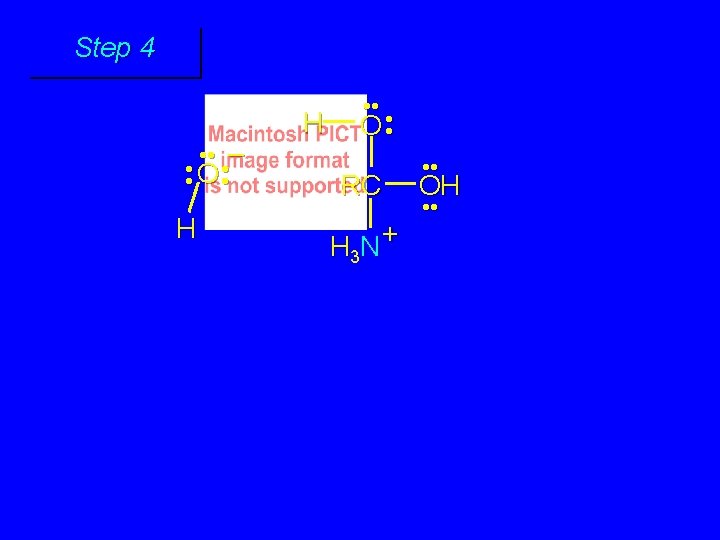
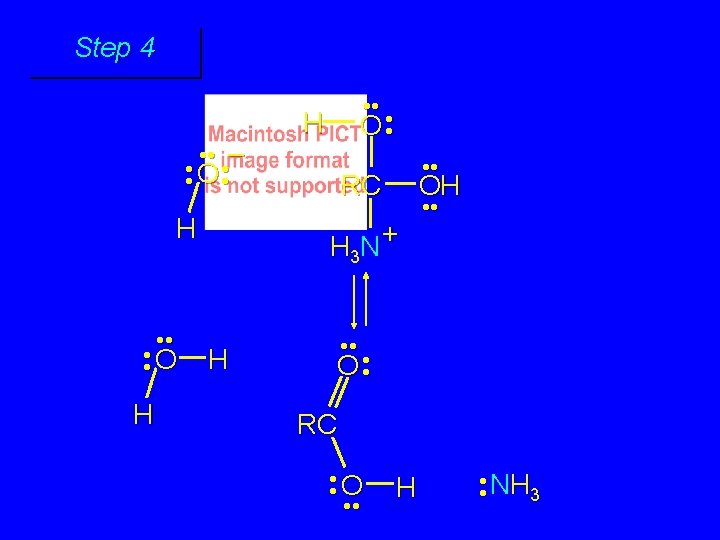
First stage: formation of tetrahedral intermediate O RCNH 2 + H 2 O HO– OH RC OH NH 2 water adds to the carbonyl group of the amide this stage is analogous to the base-catalyzed addition of water to a ketone

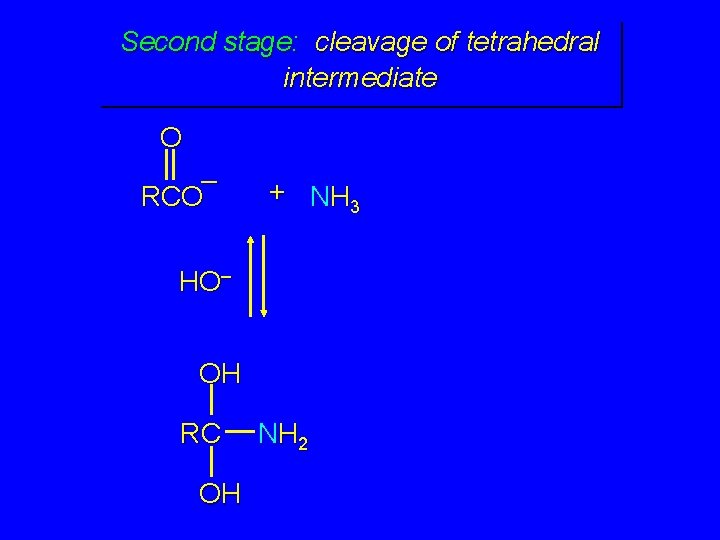
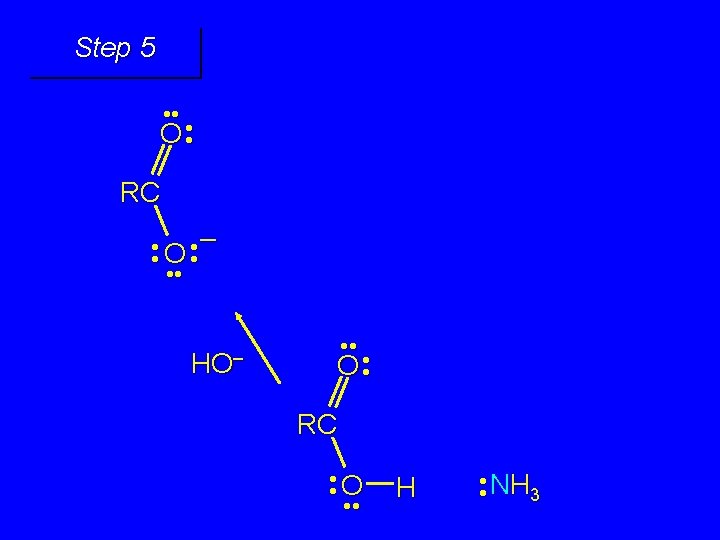
Second stage: cleavage of tetrahedral intermediate O – RCO + NH 3 HO– OH RC OH NH 2

Mechanism of formation of tetrahedral intermediate





Dissociation of tetrahedral intermediate




