2 The Citric Acid Cycle CAC Pyruvate CO
- Slides: 38

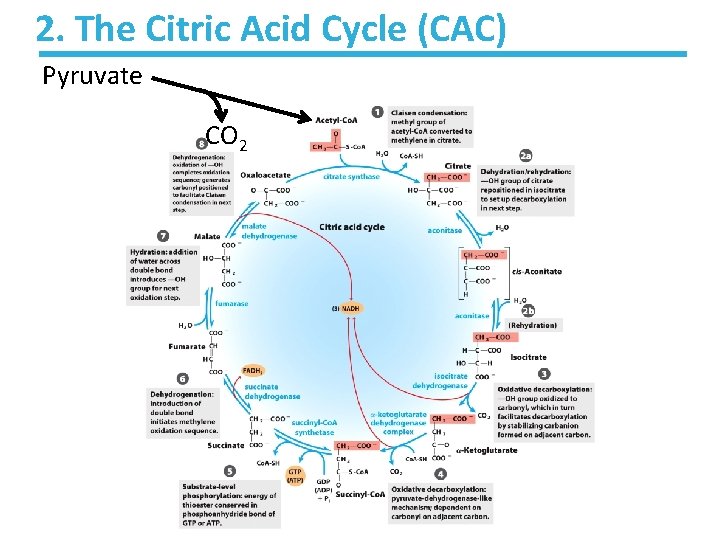
2. The Citric Acid Cycle (CAC) Pyruvate CO 2

2. The Citric Acid Cycle (CAC) The sequence of events: • Step 1: C-C bond formation to make citrate • Step 2: Isomerization via dehydration/rehydration • Steps 3– 4: Oxidative decarboxylations to give 2 NADH • Step 5: Substrate-level phosphorylation to give GTP • Step 6: Dehydrogenation to give reduced FADH 2 • Step 7: Hydration • Step 8: Dehydrogenation to give NADH

2. The Citric Acid Cycle (CAC) List of enzymes involved: 1. Synthase § Catalyzes a synthesis process 2. Aconitase § A stereo-specific isomerization 3. Dehydrogenase § Removes hydrogen as H 2 4. Synthetase § Links two molecules by using the energy of cleavage of a pyrophosphate group 5. Fumarase § Catalyzes reversible hydration/rehydration of fumarate to malate

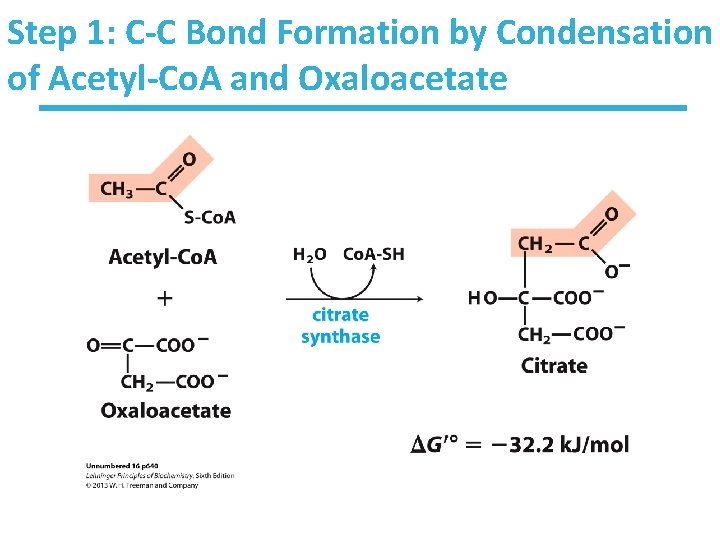
Step 1: C-C Bond Formation by Condensation of Acetyl-Co. A and Oxaloacetate

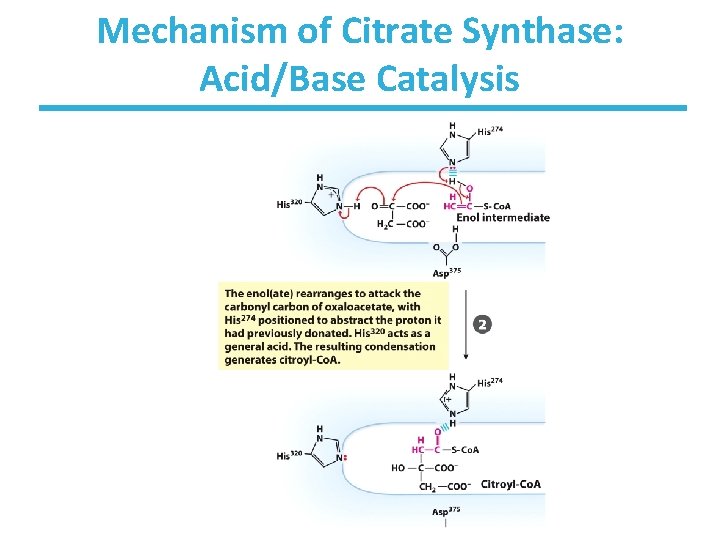
Citrate Synthase Reaction • Condensation of acetyl-Co. A and oxaloacetate • The only reaction with C-C bond formation • Rate-limiting step of CAC Mechanism • Uses Acid/Base Catalysis – Carbonyl of oxaloacetate is a good electrophile – Methyl of acetyl-Co. A is NOT a good nucleophile but is activated by deprotonation Highly thermodynamically favorable/irreversible – Regulated by substrate availability and product inhibition – Activity largely depends on [oxaloacetate]

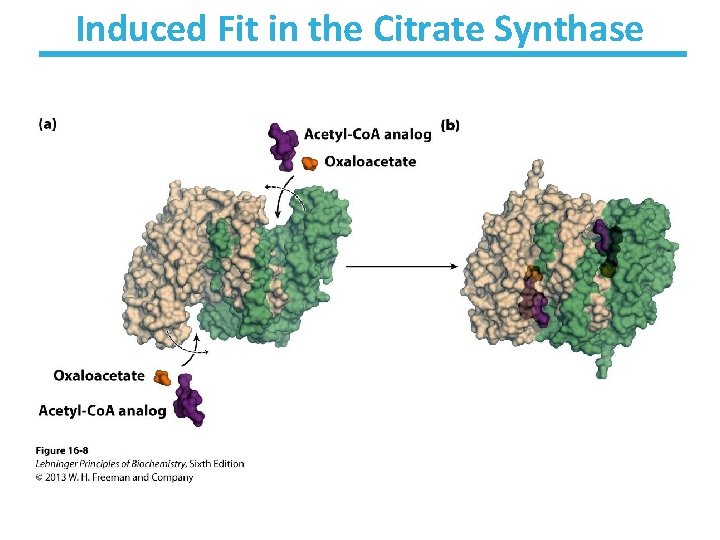
Induced Fit in the Citrate Synthase has two subunits that create two binding sites for binding both oxaloacetate and acetyl-Co. A. Binding is very conformation dependent: A. Open conformation § Free enzyme does not have a binding site for acetyl-Co. A B. Closed conformation § Binding of OAA enables binding for acetyl-Co. A § The conformation avoids hydrolysis of thioester in acetyl-Co. A § Protects reactive carbanion

Induced Fit in the Citrate Synthase

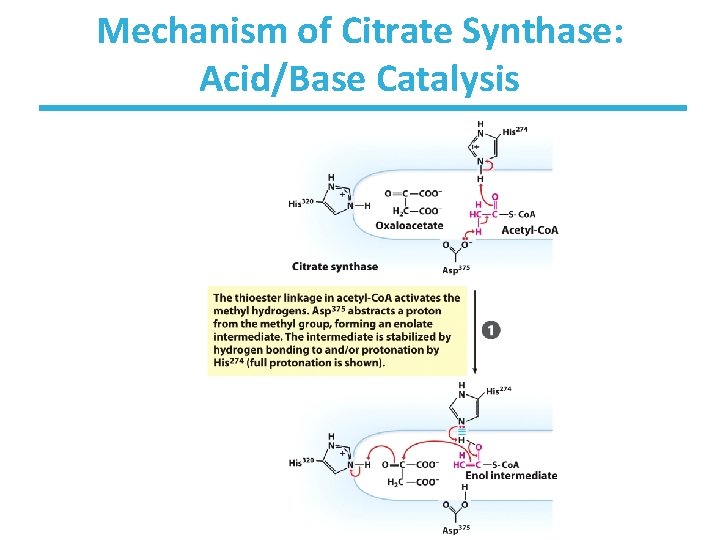
Mechanism of Citrate Synthase: Acid/Base Catalysis

Mechanism of Citrate Synthase: Acid/Base Catalysis

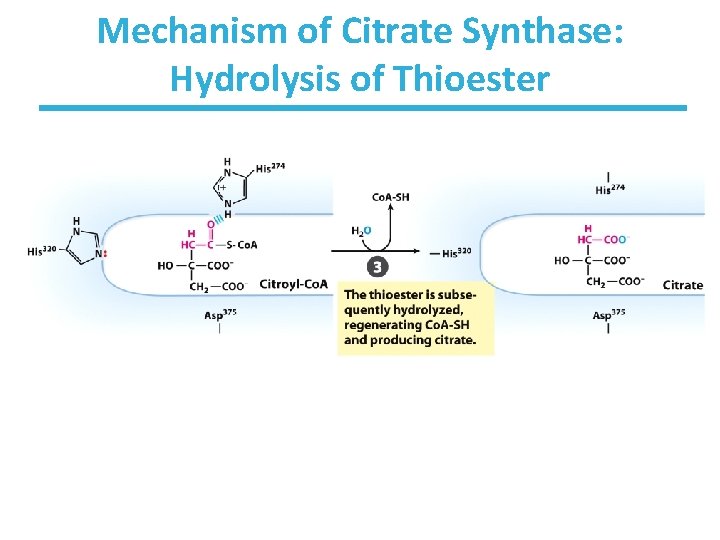
Mechanism of Citrate Synthase: Hydrolysis of Thioester

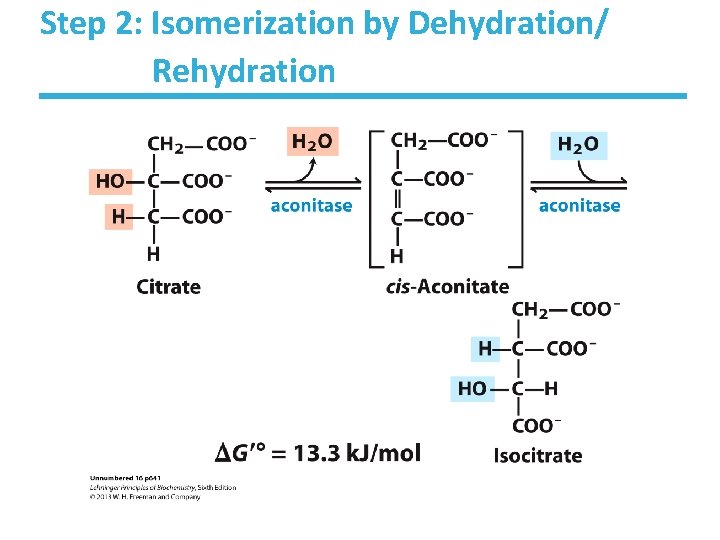
Step 2: Isomerization by Dehydration/ Rehydration

Aconitase Key points: • Elimination of H 2 O from citrate gives a cis C=C bond – Lyase • Citrate, a tertiary alcohol, is a poor substrate for oxidation – Isocitrate, a secondary alcohol, is a good substrate for oxidation • Addition of H 2 O to cis-aconitate is stereospecific • Thermodynamically unfavorable/reversible – Product concentration kept low to pull forward

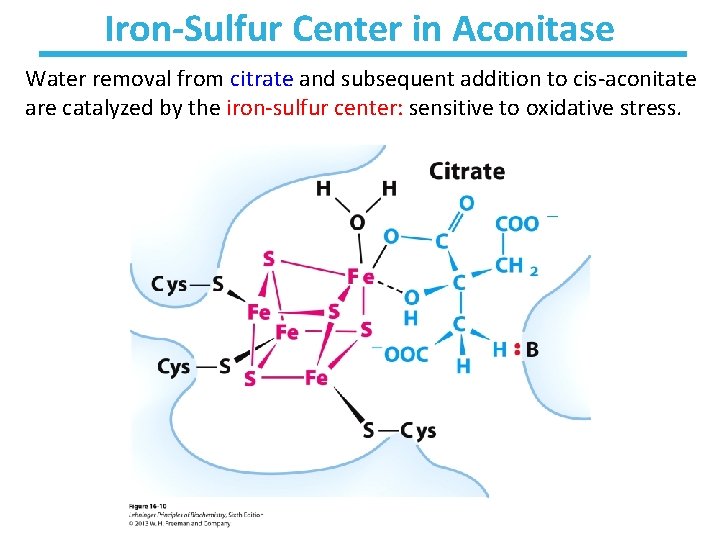
Iron-Sulfur Center in Aconitase Water removal from citrate and subsequent addition to cis-aconitate are catalyzed by the iron-sulfur center: sensitive to oxidative stress.

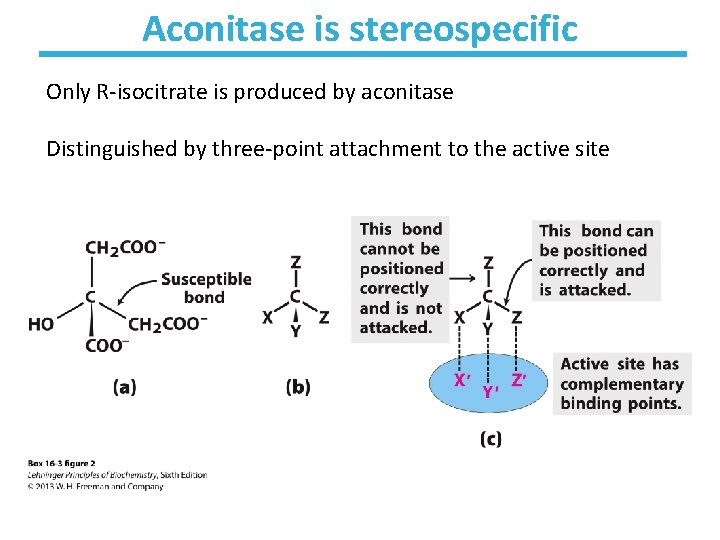
Aconitase is stereospecific Only R-isocitrate is produced by aconitase Distinguished by three-point attachment to the active site

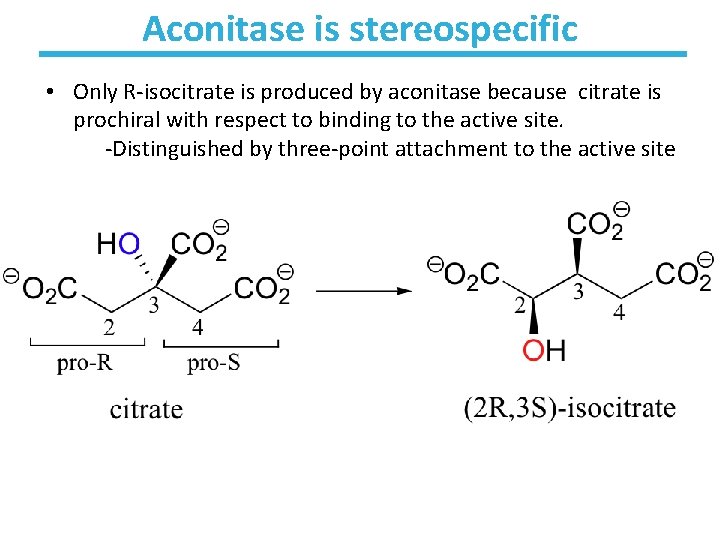
Aconitase is stereospecific • Only R-isocitrate is produced by aconitase because citrate is prochiral with respect to binding to the active site. -Distinguished by three-point attachment to the active site

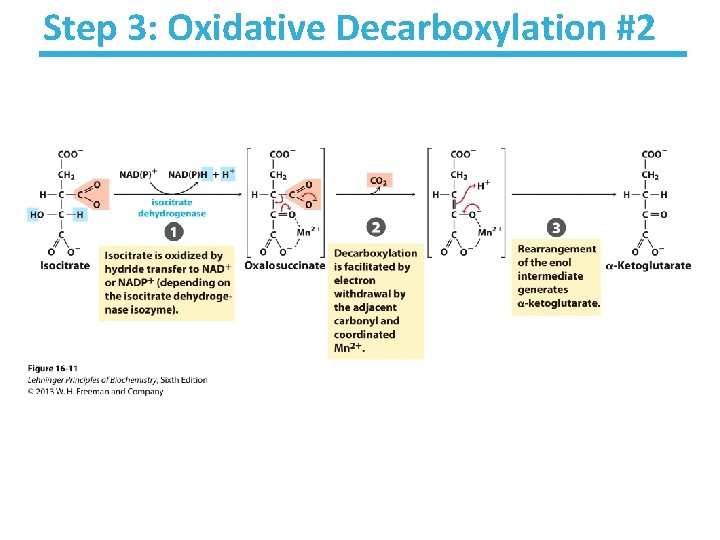
Step 3: Oxidative Decarboxylation #2

Isocitrate Dehydrogenase Key points: • Oxidative decarboxylation – Lose a carbon as CO 2 – Oxidation of the alcohol to a ketone – Transfers a hydride to NAD+ generating NADH • Cytosolic isozyme uses NADP+ as a cofactor • Highly thermodynamically favorable/irreversible – Regulated by product inhibition and ATP

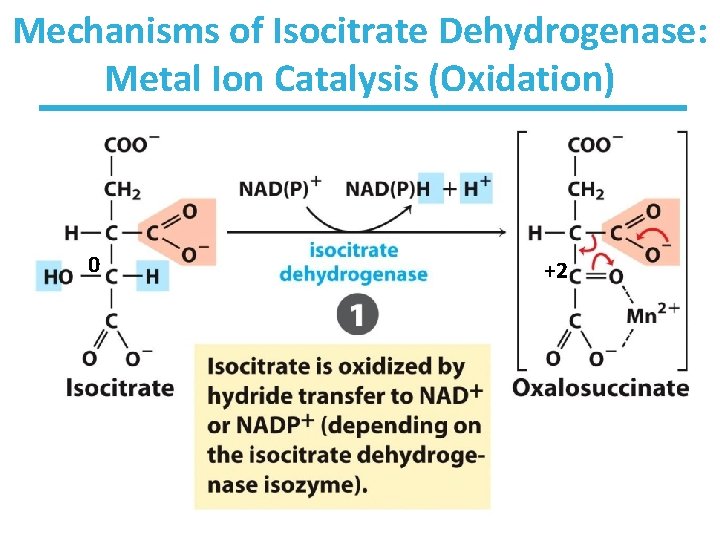
Mechanisms of Isocitrate Dehydrogenase: Metal Ion Catalysis (Oxidation) 0 +2

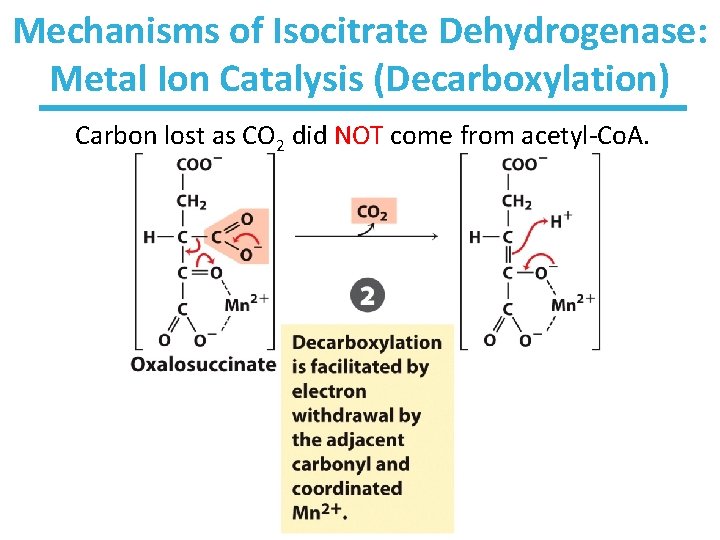
Mechanisms of Isocitrate Dehydrogenase: Metal Ion Catalysis (Decarboxylation) Carbon lost as CO 2 did NOT come from acetyl-Co. A.

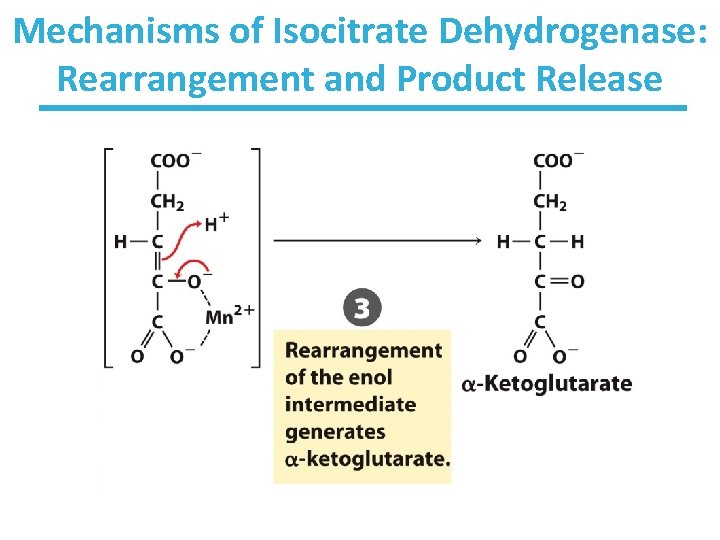
Mechanisms of Isocitrate Dehydrogenase: Rearrangement and Product Release

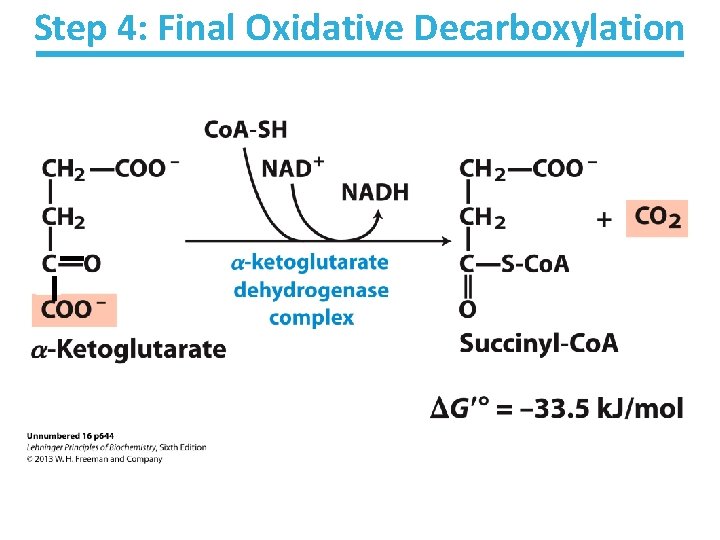
Step 4: Final Oxidative Decarboxylation

-Ketoglutarate Dehydrogenase Key points: • Last oxidative decarboxylation – Net full oxidation of all carbons of glucose • Carbons not directly from glucose because carbons lost came from oxaloacetate • Succinyl-Co. A is another higher-energy thioester bond • Highly thermodynamically favorable/irreversible – Regulated by product inhibition

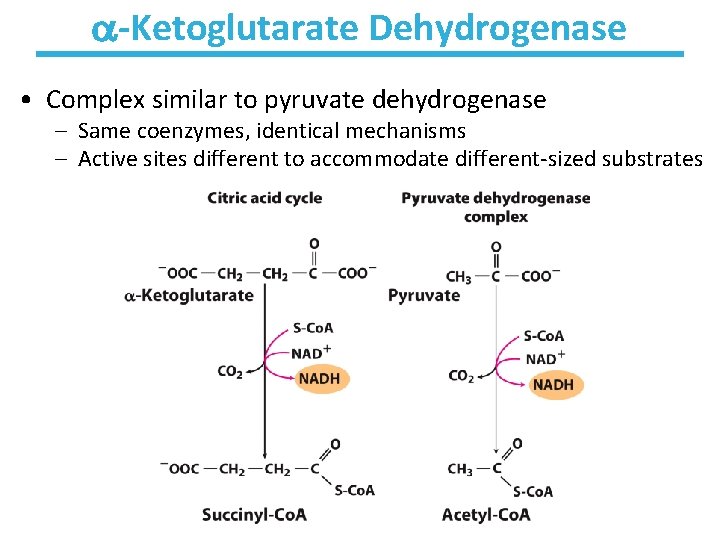
-Ketoglutarate Dehydrogenase • Complex similar to pyruvate dehydrogenase – Same coenzymes, identical mechanisms – Active sites different to accommodate different-sized substrates

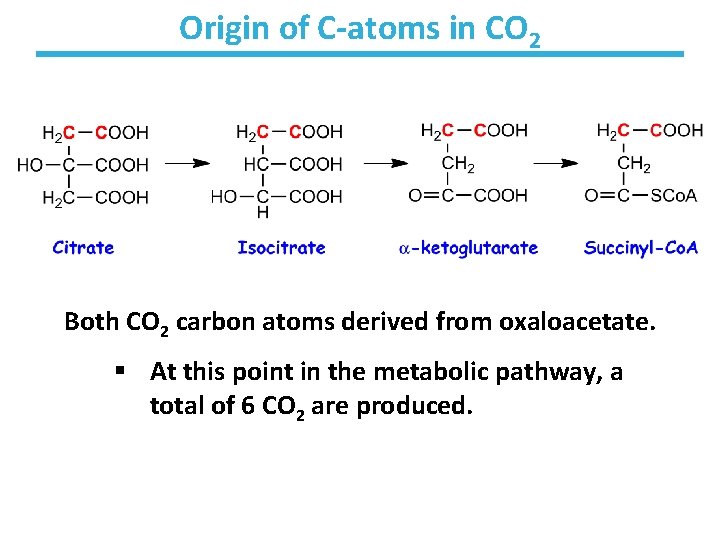
Origin of C-atoms in CO 2 Both CO 2 carbon atoms derived from oxaloacetate. § At this point in the metabolic pathway, a total of 6 CO 2 are produced.

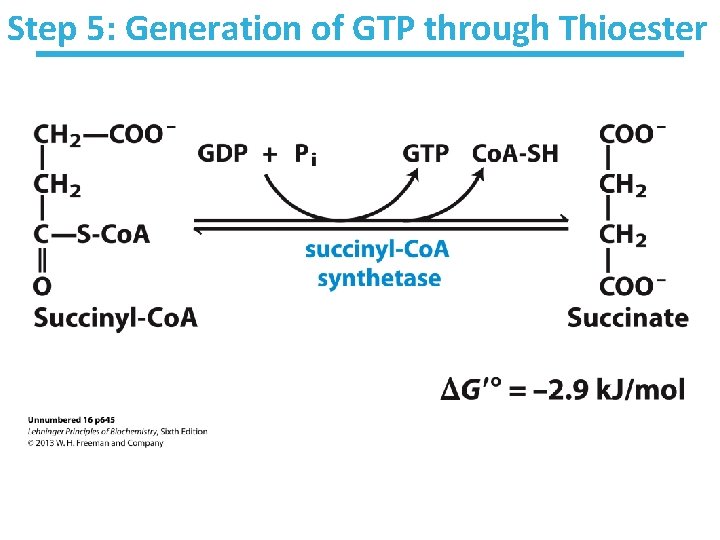
Step 5: Generation of GTP through Thioester

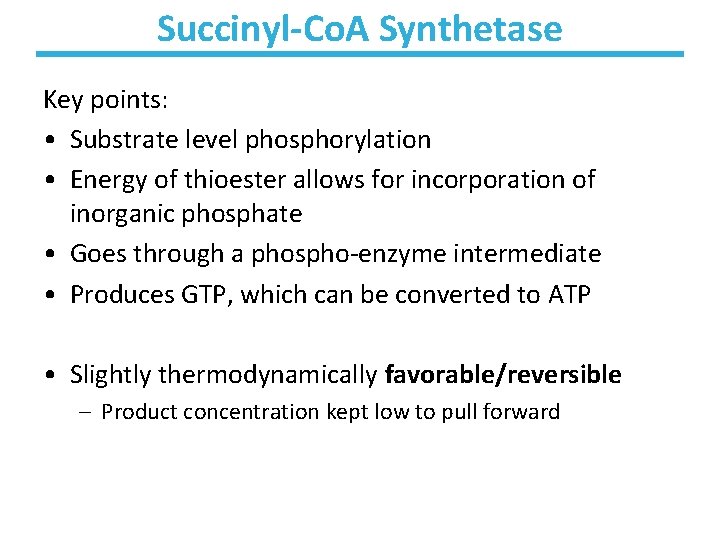
Succinyl-Co. A Synthetase Key points: • Substrate level phosphorylation • Energy of thioester allows for incorporation of inorganic phosphate • Goes through a phospho-enzyme intermediate • Produces GTP, which can be converted to ATP • Slightly thermodynamically favorable/reversible – Product concentration kept low to pull forward

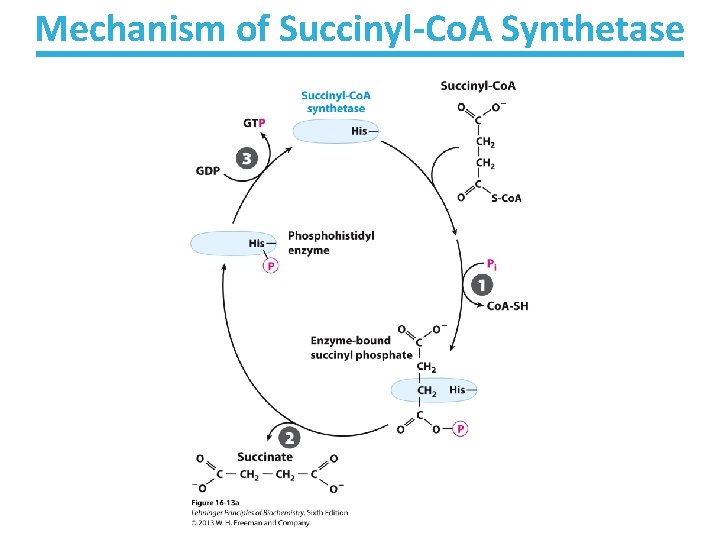
Mechanism of Succinyl-Co. A Synthetase

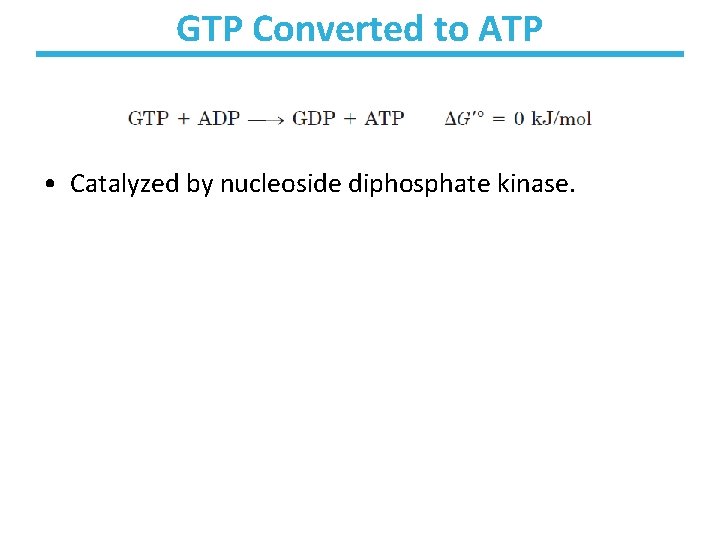
GTP Converted to ATP • Catalyzed by nucleoside diphosphate kinase.

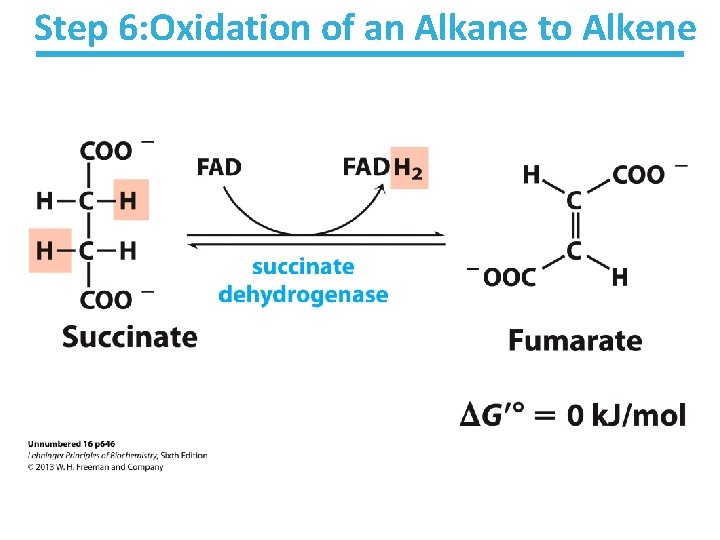
Step 6: Oxidation of an Alkane to Alkene

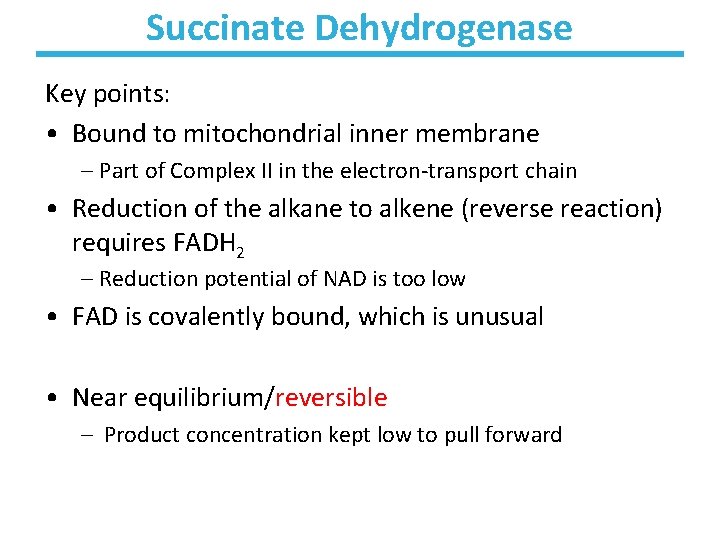
Succinate Dehydrogenase Key points: • Bound to mitochondrial inner membrane – Part of Complex II in the electron-transport chain • Reduction of the alkane to alkene (reverse reaction) requires FADH 2 – Reduction potential of NAD is too low • FAD is covalently bound, which is unusual • Near equilibrium/reversible – Product concentration kept low to pull forward

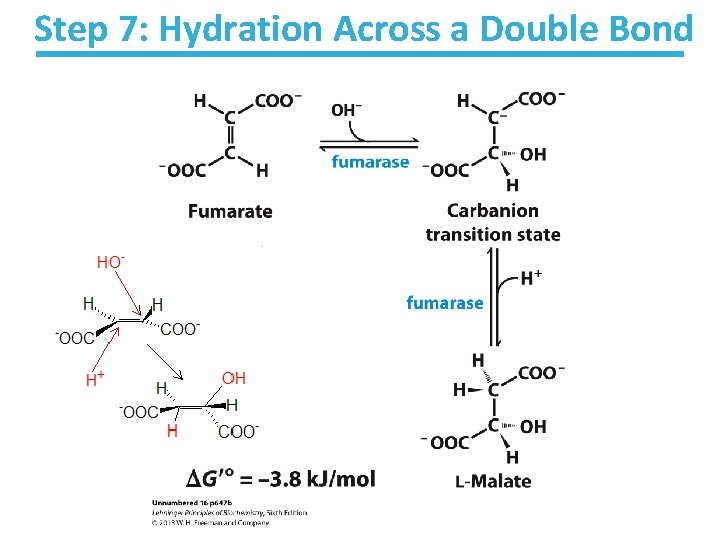
Step 7: Hydration Across a Double Bond

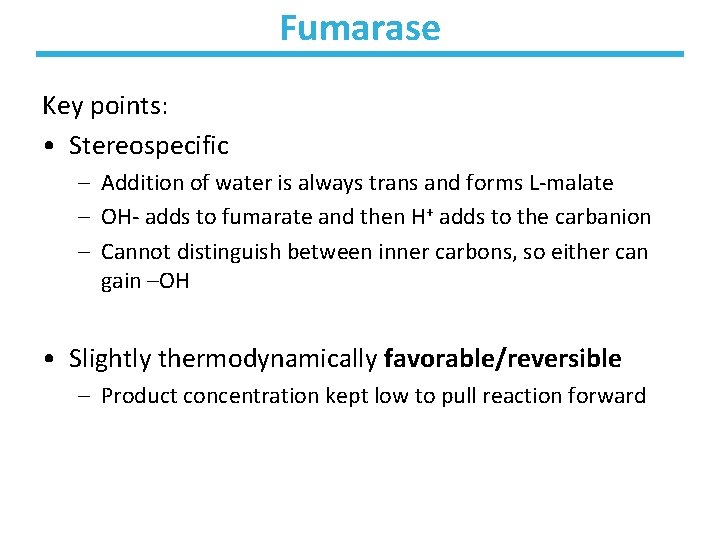
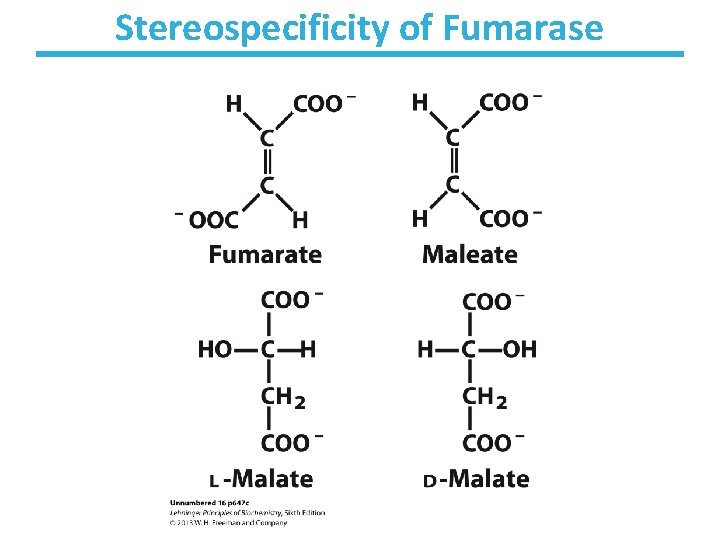
Fumarase Key points: • Stereospecific – Addition of water is always trans and forms L-malate – OH- adds to fumarate and then H+ adds to the carbanion – Cannot distinguish between inner carbons, so either can gain –OH • Slightly thermodynamically favorable/reversible – Product concentration kept low to pull reaction forward

Stereospecificity of Fumarase

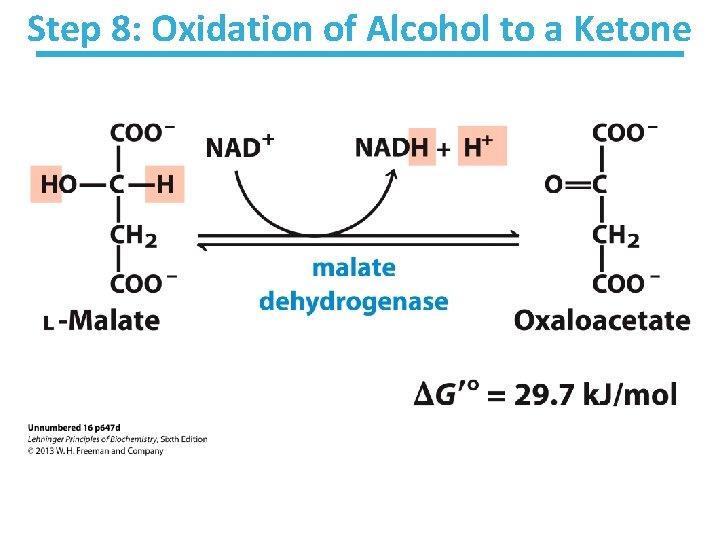
Step 8: Oxidation of Alcohol to a Ketone

Malate Dehydrogenase Key points: • Final step of the cycle • Regenerates oxaloacetate for citrate synthase • Highly thermodynamically UNfavorable/reversible – Oxaloacetate concentration kept VERY low by citrate synthase • Pulls the reaction forward

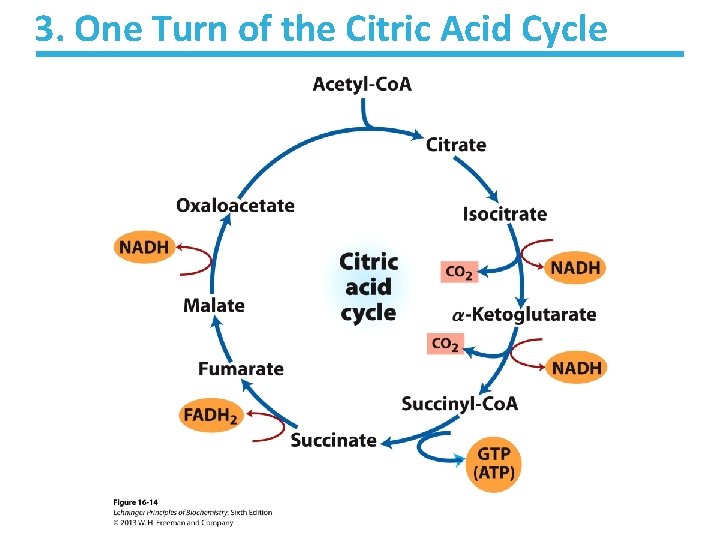
3. One Turn of the Citric Acid Cycle

3 A. Net Result of the Citric Acid Cycle Acetyl-Co. A + 3 NAD+ + FAD + GDP + Pi + 2 H 2 O 2 CO 2 + 3 NADH + FADH 2 + GTP + Co. A + 3 H+ • Net oxidation of two carbons to CO 2 – Equivalent to two carbons of acetyl-Co. A – but NOT the exact same carbons • Energy captured by electron transfer to NADH and FADH 2 • Generates 1 GTP, which can be converted to ATP

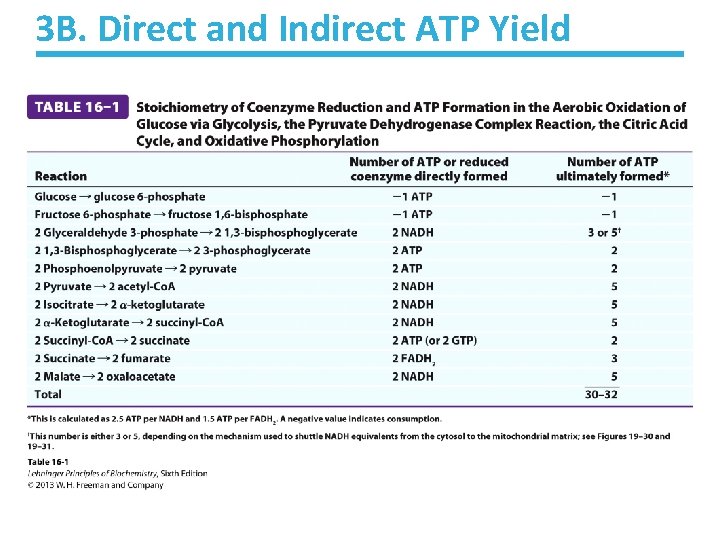
3 B. Direct and Indirect ATP Yield