2 Proteins 1 A polypeptide is a polymer



- Slides: 15



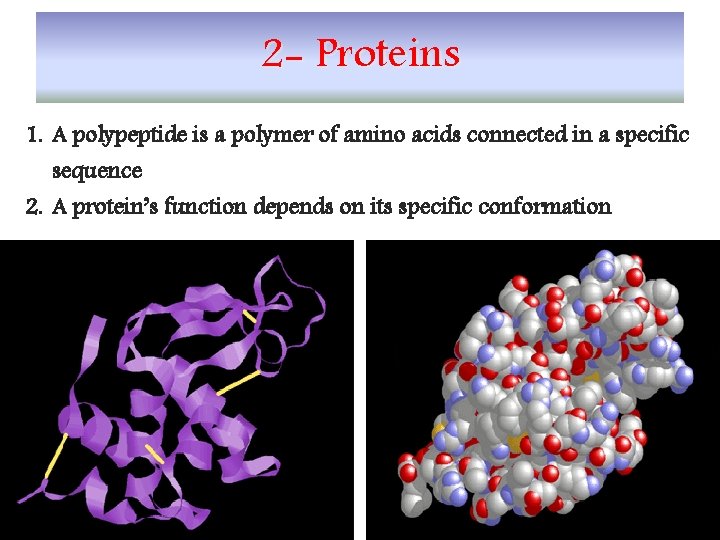
2 - Proteins 1. A polypeptide is a polymer of amino acids connected in a specific sequence 2. A protein’s function depends on its specific conformation 3

2 - Proteins • Their functions include structural support, storage, transport of other substances, intercellular signaling, movement, and defense against microbes. • Some proteins works as enzymes in the cell that regulate metabolism by accelerating chemical reactions. • All protein polymers are constructed from 20 monomers, called amino acids. • Polymers of proteins are called polypeptides. • A protein consists of one or more peptides (polypeptides) folded and coiled into a specific conformation 4

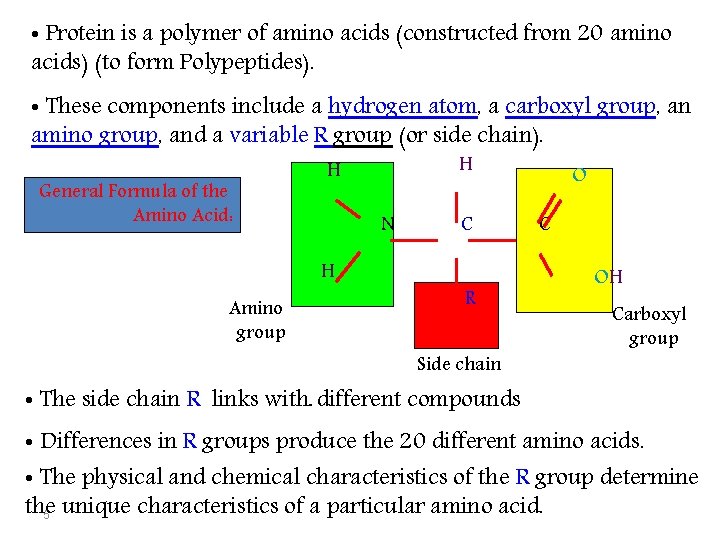
• Protein is a polymer of amino acids (constructed from 20 amino acids) (to form Polypeptides). • These components include a hydrogen atom, a carboxyl group, an amino group, and a variable R group (or side chain). General Formula of the Amino Acid: N H Amino group H H C R Side chain O C OH Carboxyl group • The side chain R links with ـ different compounds • Differences in R groups produce the 20 different amino acids. • The physical and chemical characteristics of the R group determine the 5 unique characteristics of a particular amino acid.

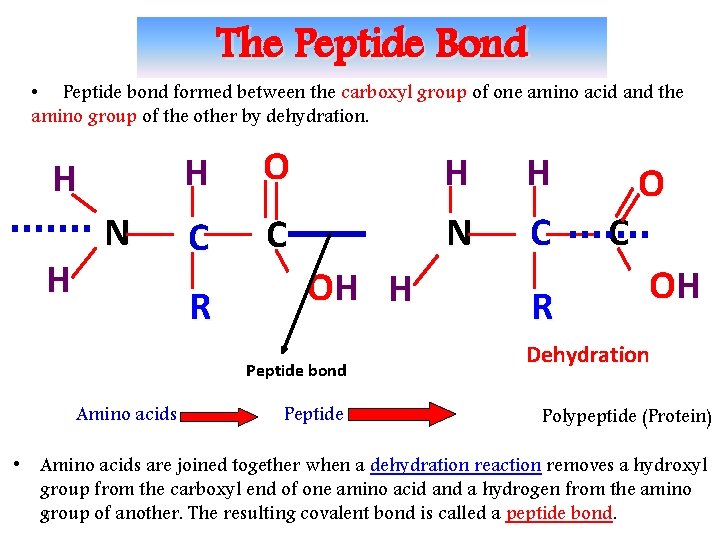
The Peptide Bond • Peptide bond formed between the carboxyl group of one amino acid and the amino group of the other by dehydration. H H N H O C C R H N OH H Peptide bond Amino acids Peptide H C R C O OH Dehydration Polypeptide (Protein) • Amino acids are joined together when a dehydration reaction removes a hydroxyl group from the carboxyl end of one amino acid and a hydrogen from the amino 6 group of another. The resulting covalent bond is called a peptide bond.

3 - Lipids The Hydrophobic Molecules 1. Fats store large amounts of energy 2. Phospholipids are major components of cell membranes 3. Steroids include cholesterol and certain hormones 7

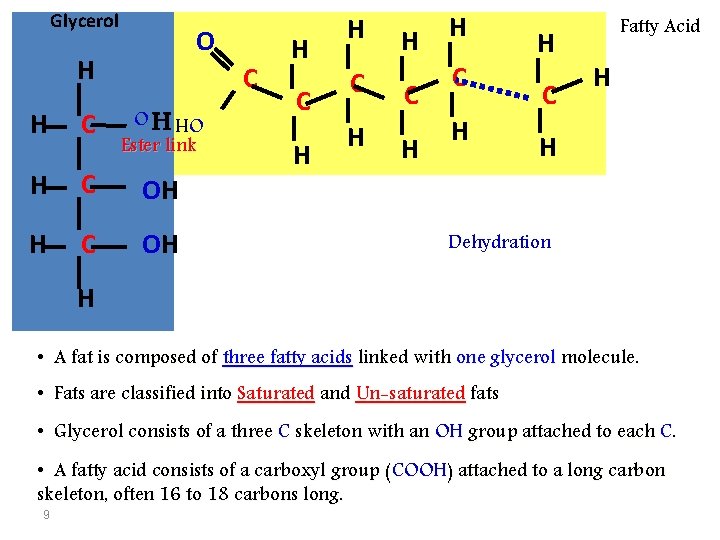
1)- Fats: • Lipids are an exception among macromolecules because they do • not have polymers. The unifying feature of lipids is that they all have little or no affinity for water. – This is because their structures are dominated by non-polar covalent bonds. • Lipids are the components of fats, and are highly diverse in • • 8 form and function. Although fats are not polymers, they are large molecules assembled from smaller molecules by dehydration reactions. A fat is constructed from two kinds of smaller molecules, glycerol and fatty acids.

Glycerol O H C OH Ester link H C OH H C H H C Fatty Acid H H Dehydration OH H • A fat is composed of three fatty acids linked with one glycerol molecule. • Fats are classified into Saturated and Un-saturated fats • Glycerol consists of a three C skeleton with an OH group attached to each C. • A fatty acid consists of a carboxyl group (COOH) attached to a long carbon skeleton, often 16 to 18 carbons long. 9

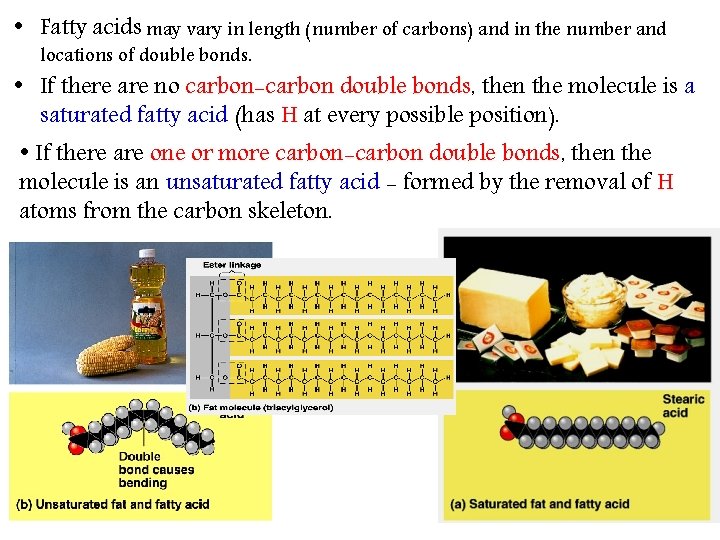
• Fatty acids may vary in length (number of carbons) and in the number and locations of double bonds. • If there are no carbon-carbon double bonds, bonds then the molecule is a saturated fatty acid (has H at every possible position). • If there are one or more carbon-carbon double bonds, bonds then the molecule is an unsaturated fatty acid - formed by the removal of H atoms from the carbon skeleton. 10

A)- Saturated Fats • The Fatty acid components are saturated (there is no double bonds between the carbons. All C are linked with H. • Thus, it is saturated with H. • Most animal fats are saturated. • They are solid at room temperature. • Saturated fats-rich diet results in Atherosclerosis. B)- Un-saturated Fats • • 11 These double bonds are formed by the removal of H atoms. Most vegetable fats (oils) and fish fats are unsaturated. They are liquid at room temperature. They can be synthetically converted to saturated (solid) by adding H (Hydrogenation).

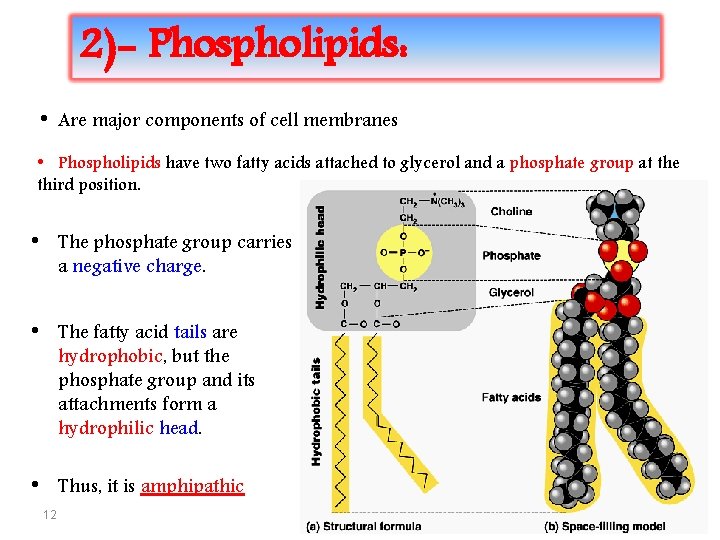
2)- Phospholipids: • Are major components of cell membranes • Phospholipids have two fatty acids attached to glycerol and a phosphate group at the third position. • The phosphate group carries a negative charge. • The fatty acid tails are hydrophobic, but the phosphate group and its attachments form a hydrophilic head. • Thus, it is amphipathic 12

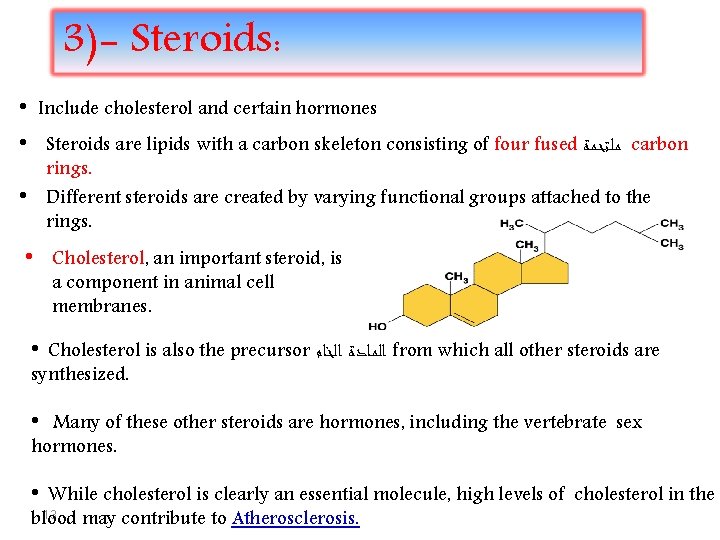
3)- Steroids: • Include cholesterol and certain hormones • Steroids are lipids with a carbon skeleton consisting of four fused ﻣﻠﺘﺤﻤﺔ carbon • rings. Different steroids are created by varying functional groups attached to the rings. • Cholesterol, an important steroid, is a component in animal cell membranes. • Cholesterol is also the precursor ﺍﻟﻤﺎﺩﺓ ﺍﻟﺨﺎﻡ from which all other steroids are synthesized. • Many of these other steroids are hormones, including the vertebrate sex hormones. • While cholesterol is clearly an essential molecule, high levels of cholesterol in the 13 blood may contribute to Atherosclerosis.

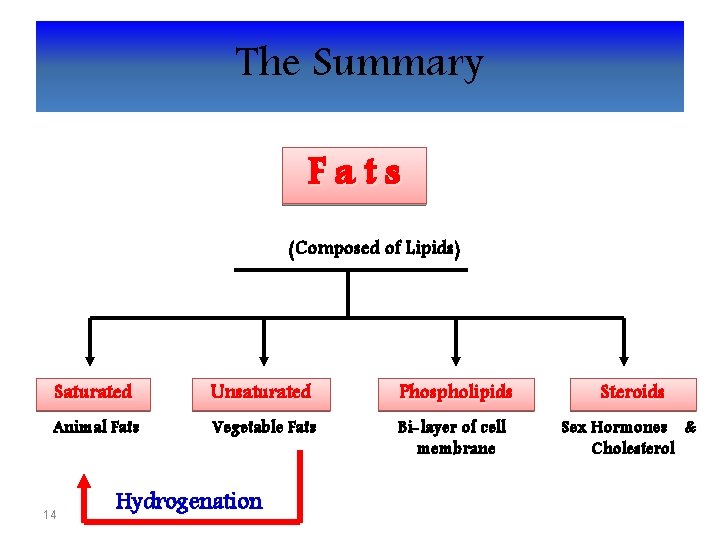
The Summary Fats (Composed of Lipids) Saturated Unsaturated Phospholipids Steroids Animal Fats Vegetable Fats Bi-layer of cell membrane Sex Hormones & Cholesterol 14 Hydrogenation
