2 DNMR Coupling Constants Geminal Coupling are usually








- Slides: 36

2 DNMR

Coupling Constants Geminal Coupling are usually negative: CH 2 Vicinal coupling usually positive: CH-CH Why? Coupling is through bonding electrons; consider the following model

2 D NMR basically provides information about connectivity and proximity COSY spectroscopy (correlation spectroscopy) (various pulse sequences are available providing similar information)

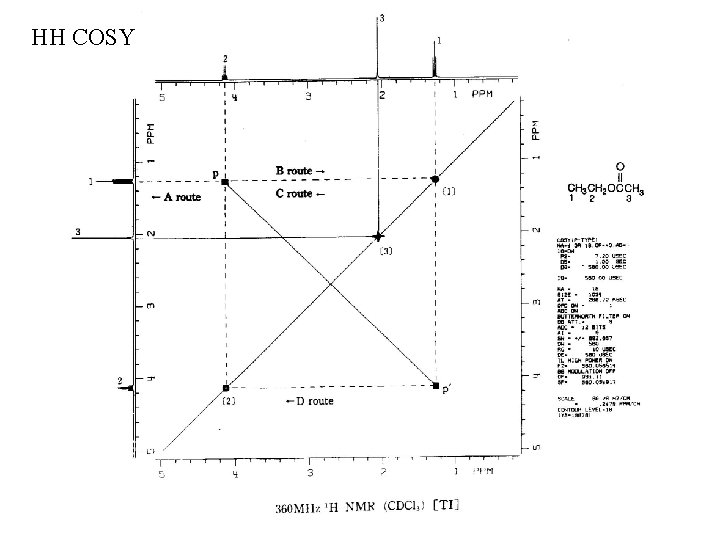
HH COSY

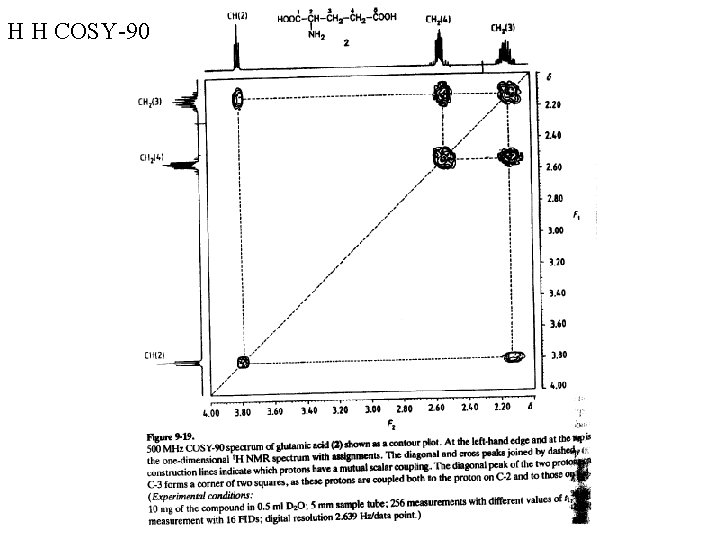
H H COSY-90

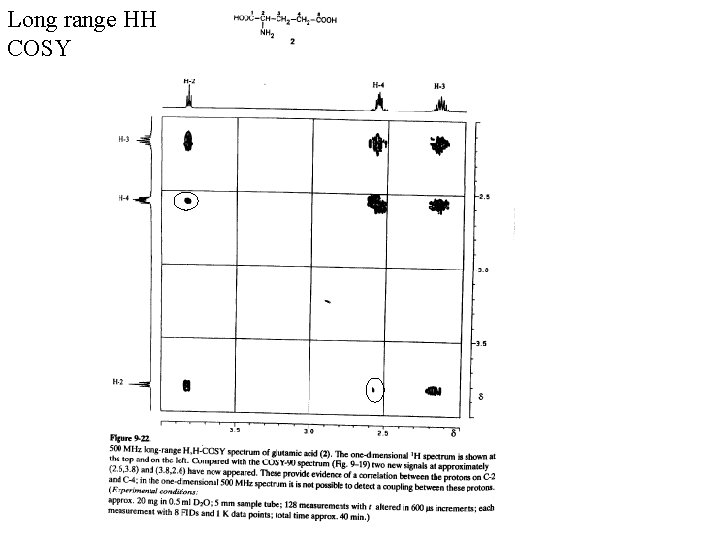
Long range HH COSY

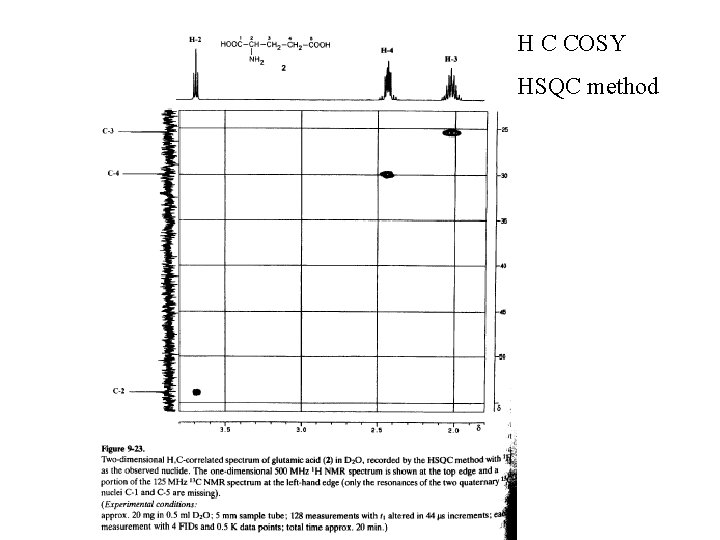
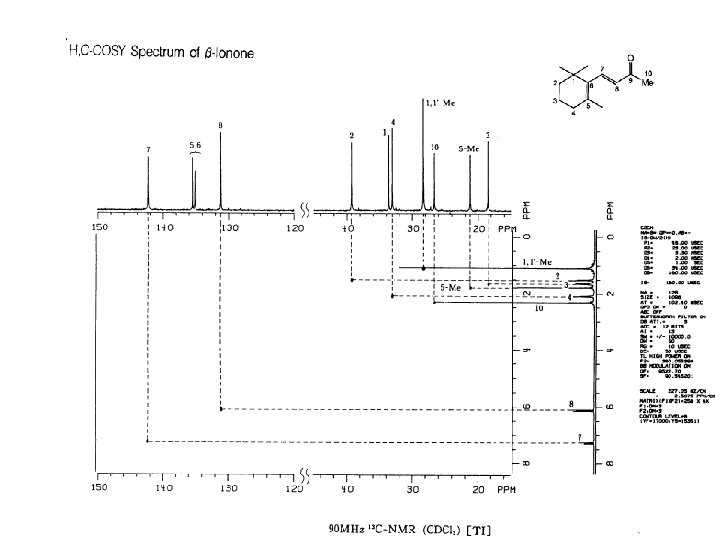
H C COSY HSQC method

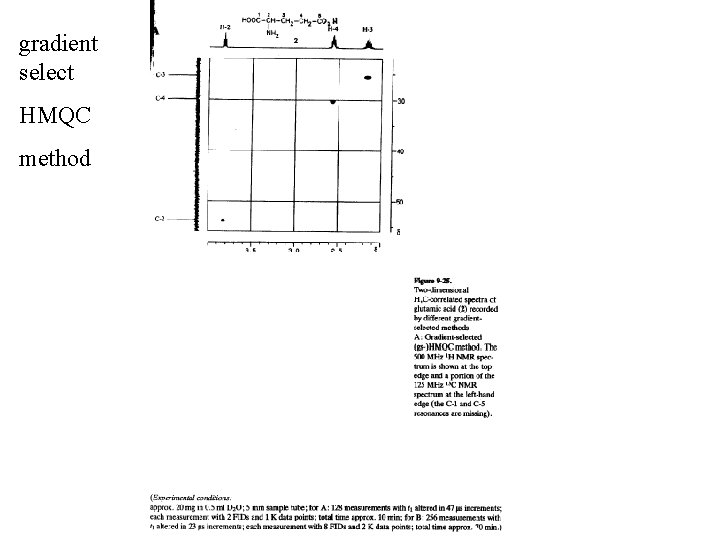
gradient select HMQC method gradient select HMBC method

2. Off-resonance decoupling

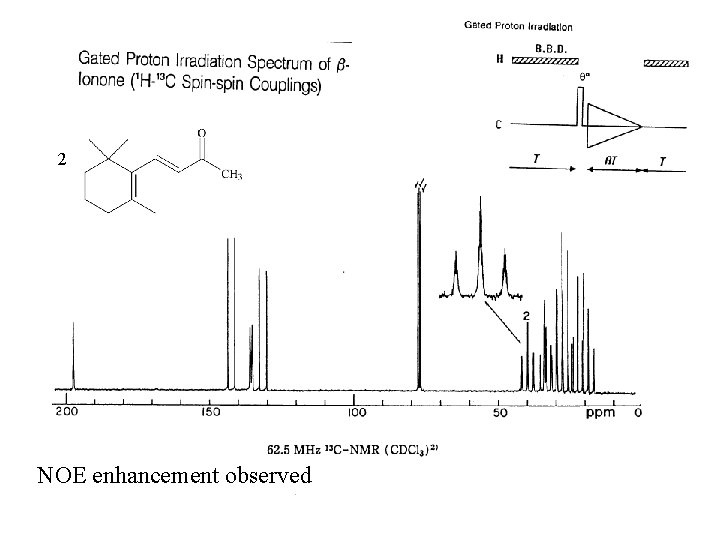
2 NOE enhancement observed

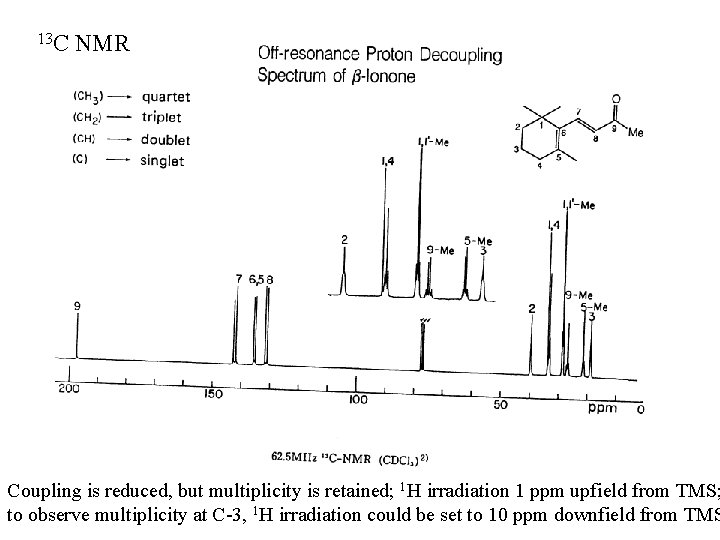
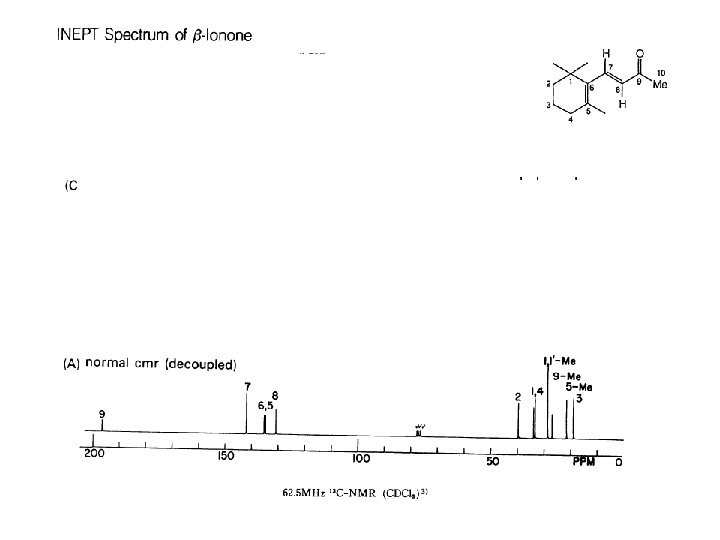
13 C NMR Coupling is reduced, but multiplicity is retained; 1 H irradiation 1 ppm upfield from TMS; to observe multiplicity at C-3, 1 H irradiation could be set to 10 ppm downfield from TMS

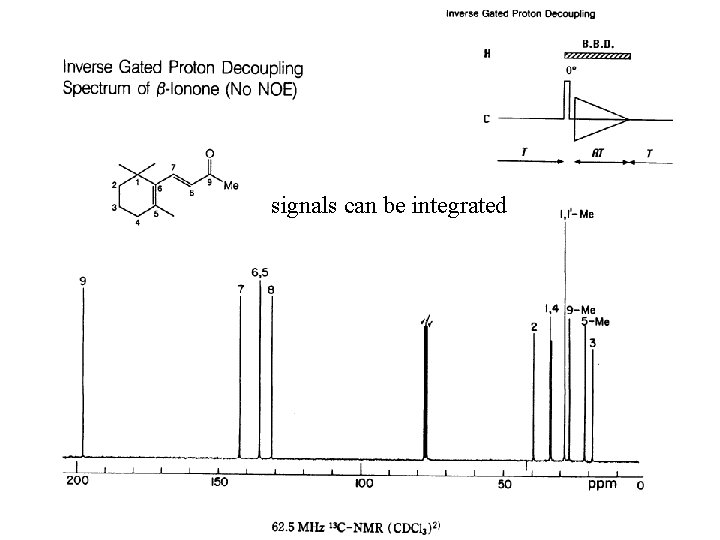
signals can be integrated

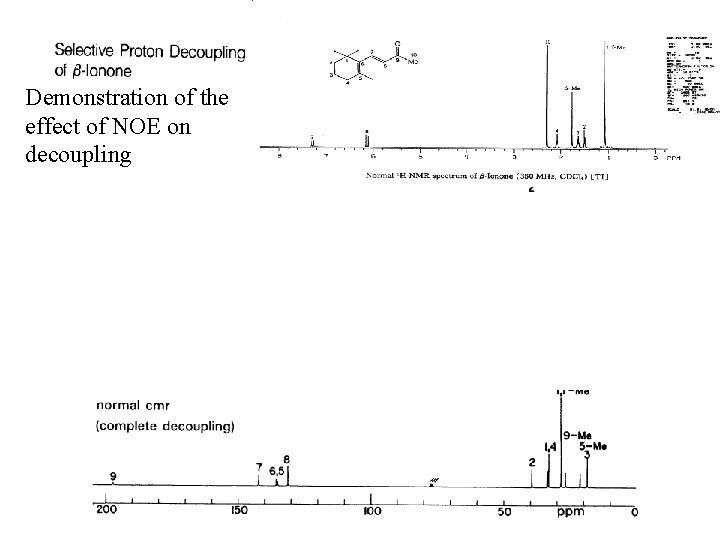
Demonstration of the effect of NOE on decoupling


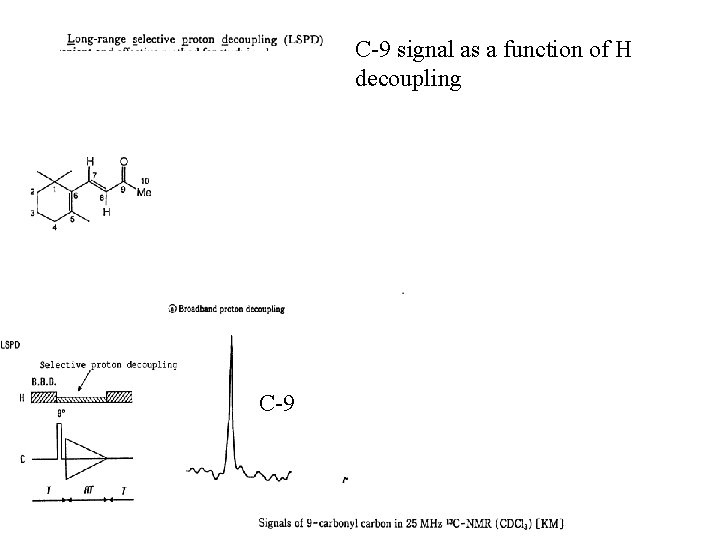
C-9 signal as a function of H decoupling C-9

3. Attached protons


4. Identification of nearest neighbors

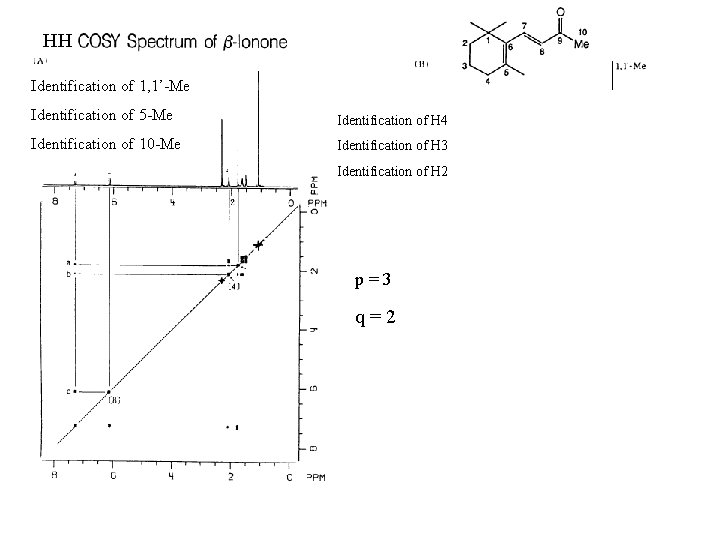
HH Identification of 1, 1’-Me Identification of 5 -Me Identification of H 4 Identification of 10 -Me Identification of H 3 Identification of H 2 p=3 q=2

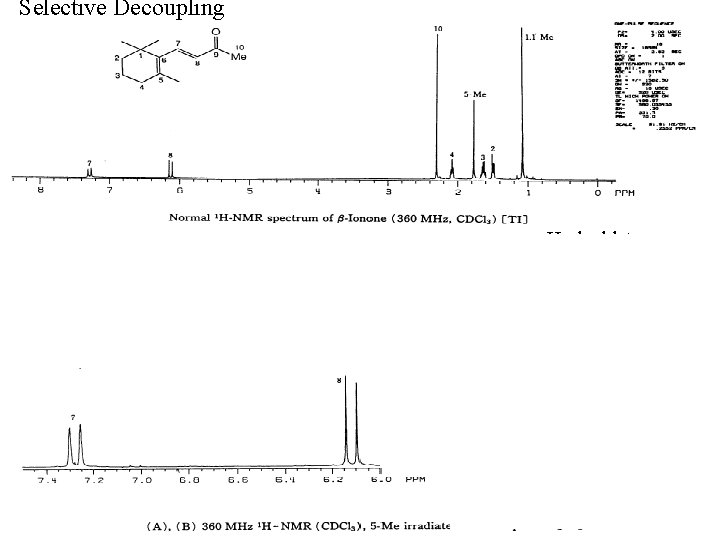
Selective Decoupling irradiate H 4 doublet triplet little interaction normal spectrum

Results are often reported as difference spectra

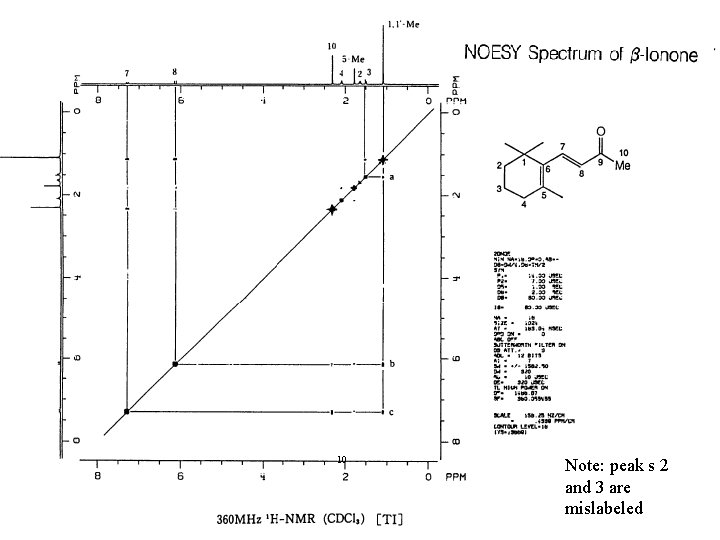
10 Note: peak s 2 and 3 are mislabeled

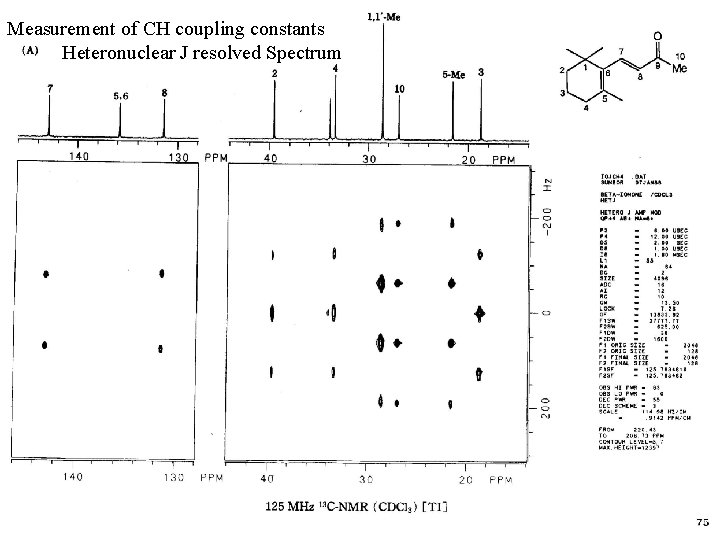
Measurement of CH coupling constants Heteronuclear J resolved Spectrum

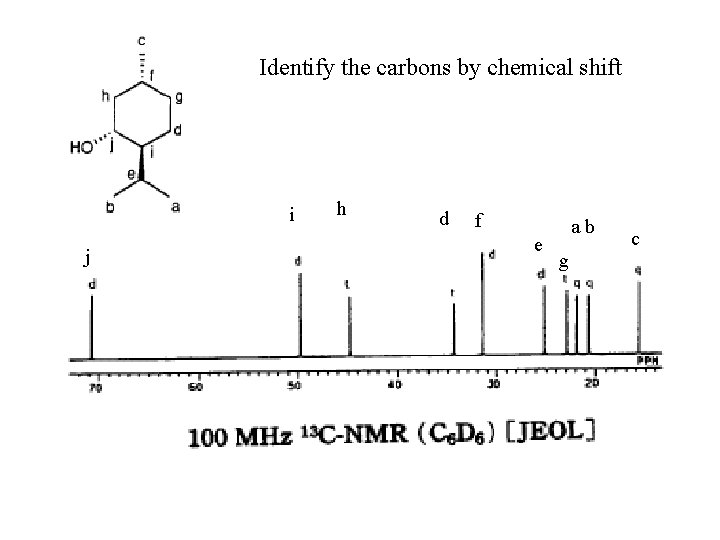
Identify the carbons by chemical shift i j h d f e ab g c

Let test our assignments using ACD

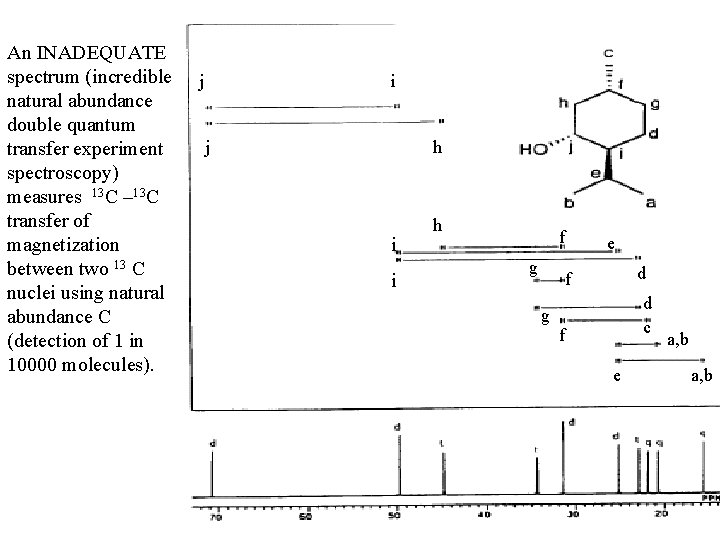
An INADEQUATE spectrum (incredible natural abundance double quantum transfer experiment spectroscopy) measures 13 C – 13 C transfer of magnetization between two 13 C nuclei using natural abundance C (detection of 1 in 10000 molecules). j i j h i i h f g e d f g d c f e a, b

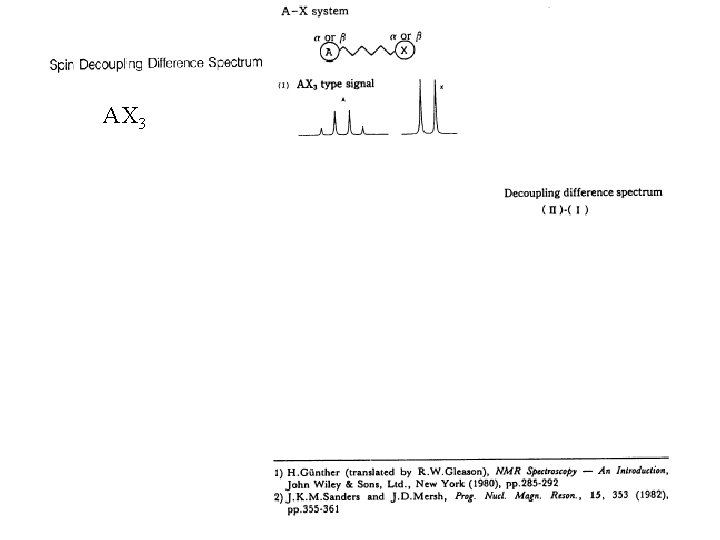
AX 3 XAM 2 where JAX= JAM JXM = 0

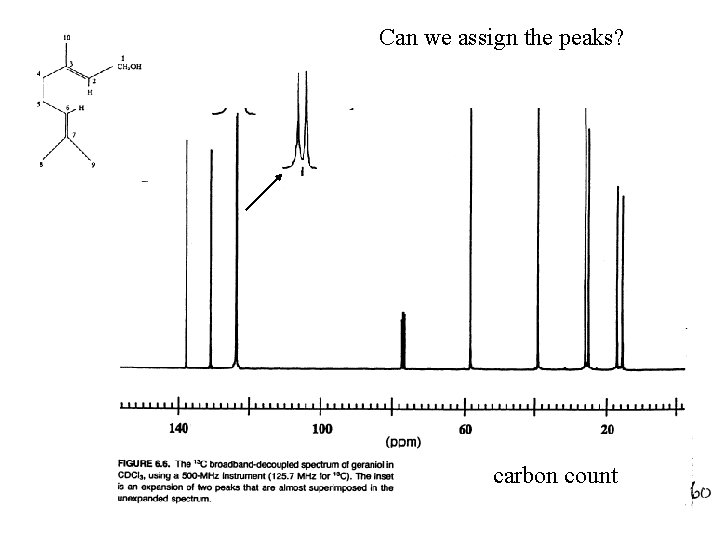
Can we assign the peaks? carbon count

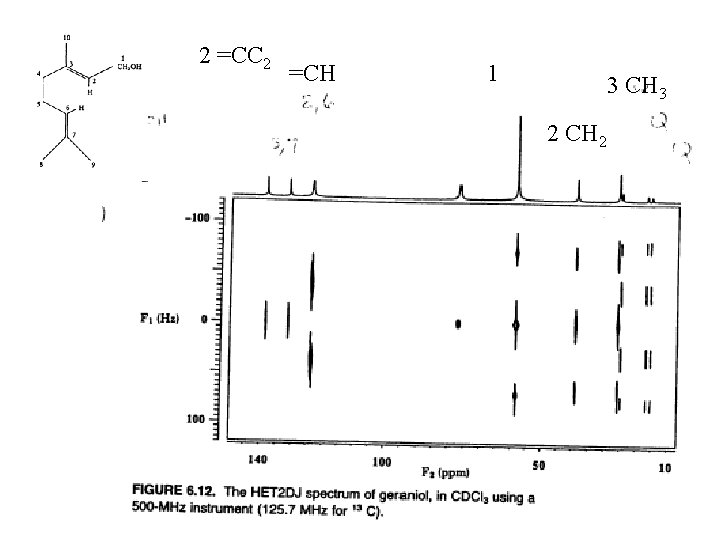
2 =CC 2 =CH 1 3 CH 3 2 CH 2

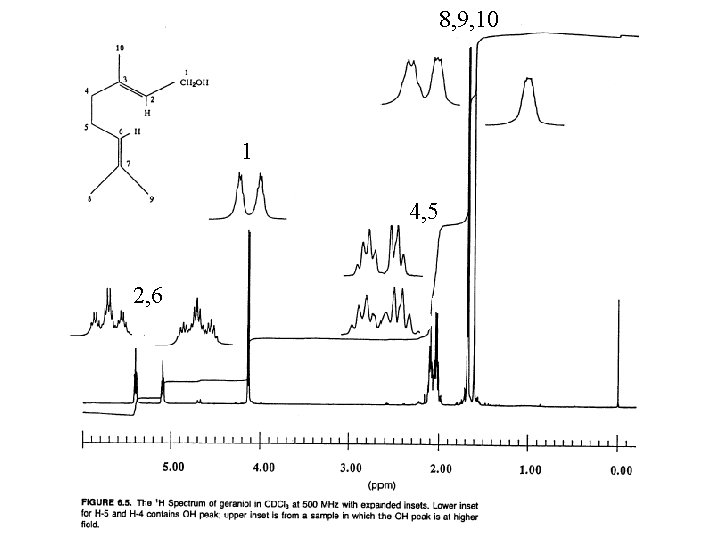
8, 9, 10 1 4, 5 2, 6

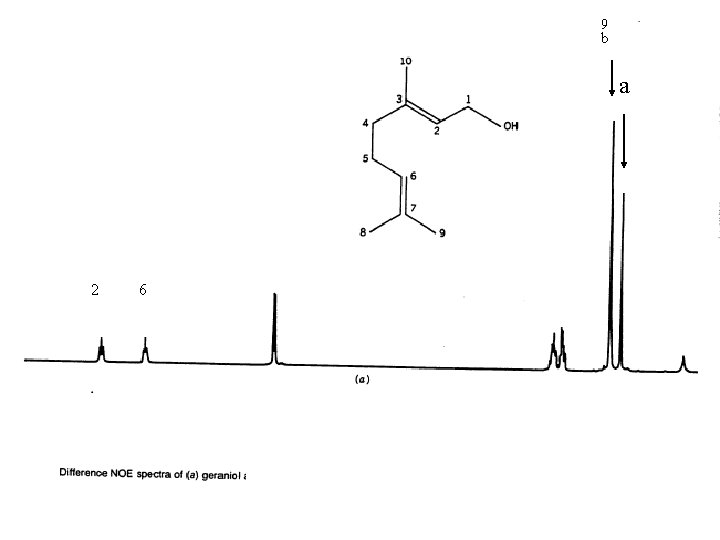
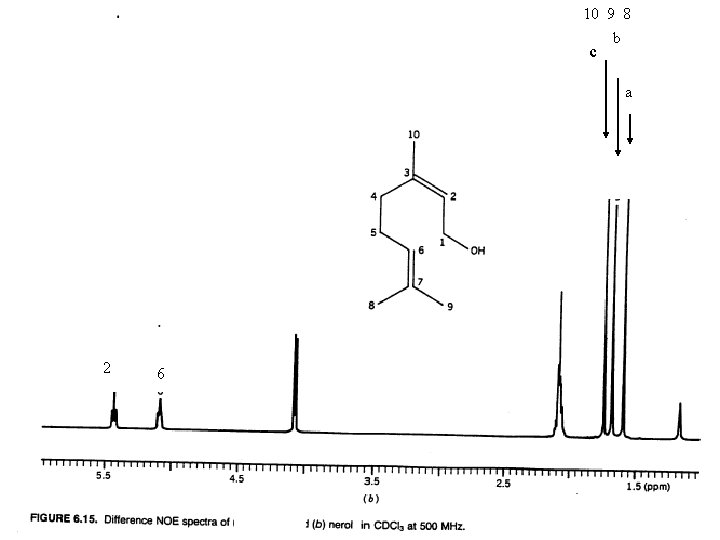
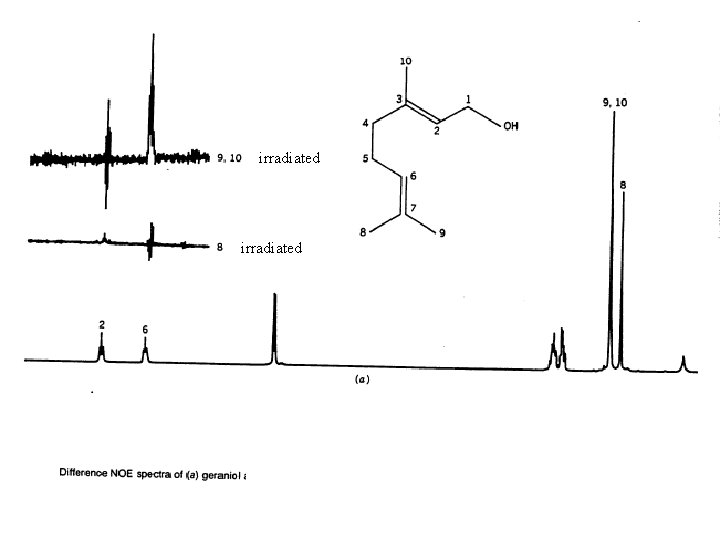
NOE Difference Spectra Some NOE operates through space as well as through bonding electrons. The through space interaction decreases as the inverse of the sixth power of the through space distance of the nuclei. The through space interaction occurs between nuclei that interact by a dipolar interaction The NOE difference spectrum is obtained by subtracting a normal spectrum from one in which a specific proton is irradiated. An measurable interaction can be expected up to about 4Å.

9 b a bb a 2 6 irradiated

10 9 8 b c a irradiated 2 6 c Irradiated b irradiated a

irradiated

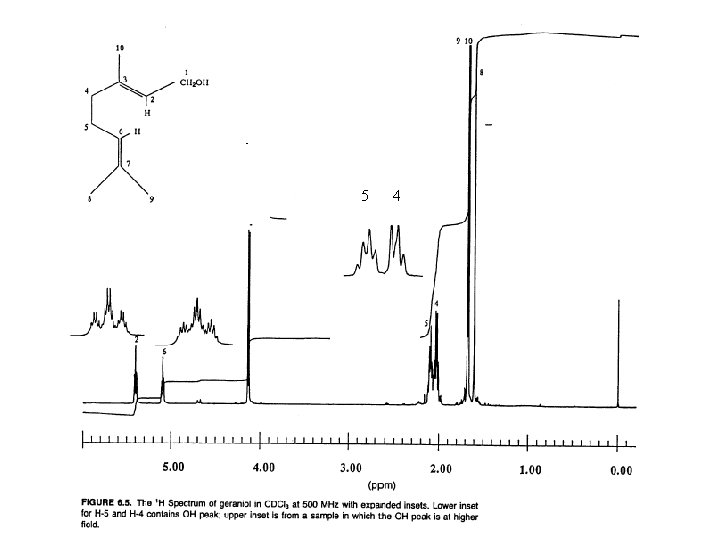
1 5 4

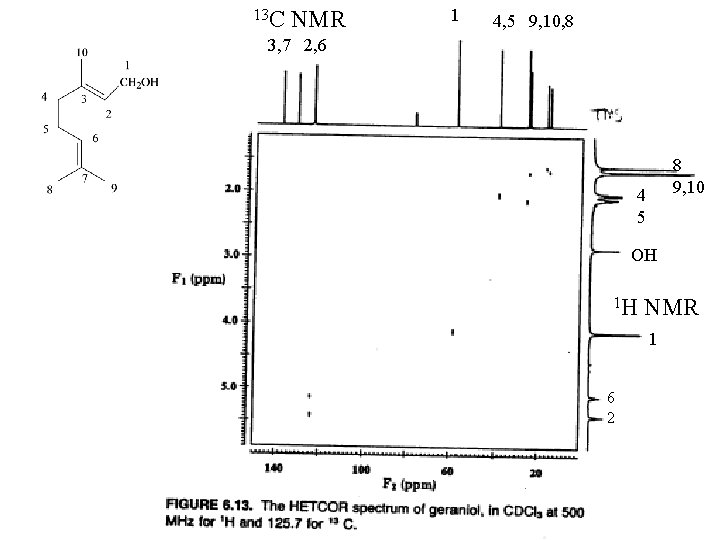
13 C NMR 1 4, 5 9, 10, 8 3, 7 2, 6 8 9, 10 4 5 OH 1 H NMR 1 6 2