2 Atomic Structure Leaving Certificate Chemistry Atoms The












- Slides: 54

2 – Atomic Structure Leaving Certificate Chemistry


Atoms • The word atom comes from the Greek word atomos…meaning unsplittable.

The Law of Conservation of mass

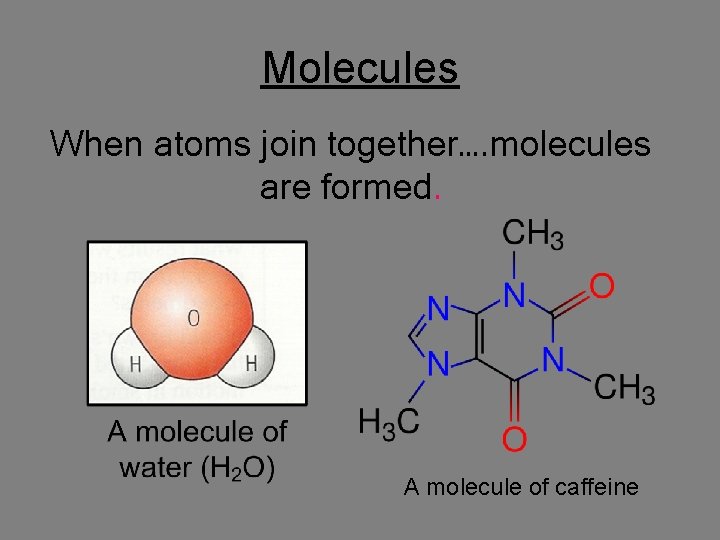
Molecules When atoms join together…. molecules are formed. A molecule of caffeine

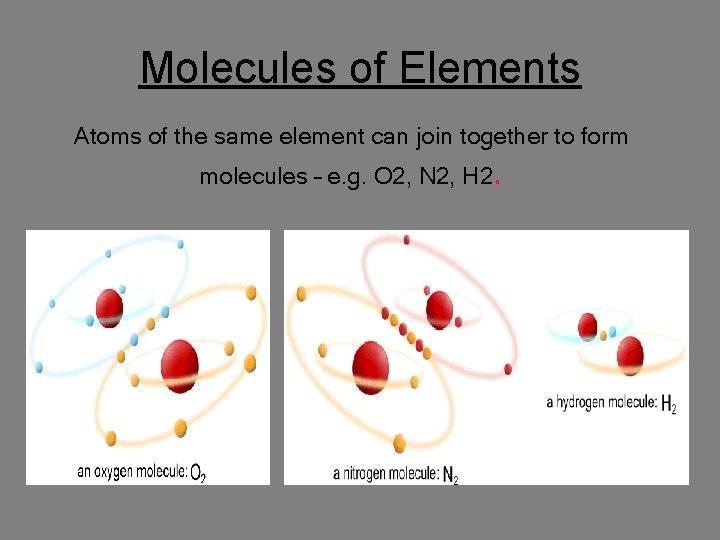
Molecules of Elements Atoms of the same element can join together to form molecules – e. g. O 2, N 2, H 2.

Ions If an atom loses or gains one or more electrons then it is called an ion.

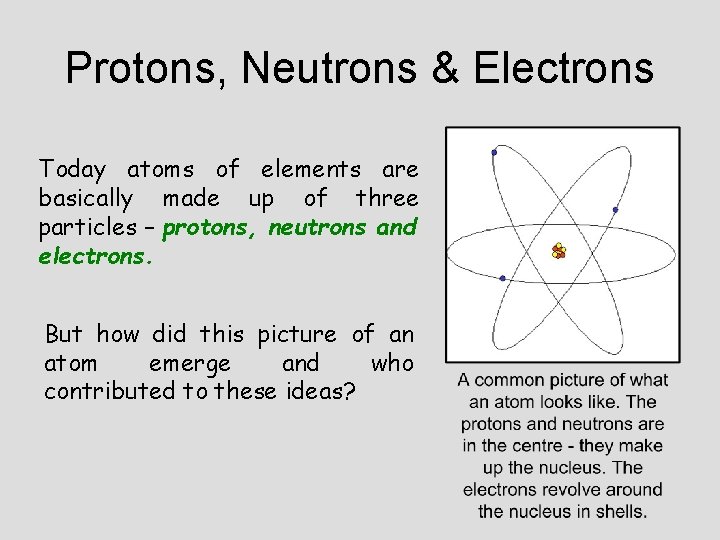
Protons, Neutrons & Electrons Today atoms of elements are basically made up of three particles – protons, neutrons and electrons. But how did this picture of an atom emerge and who contributed to these ideas?


John Dalton’s theory: 1. Every chemical element is made up of atoms of a unique type. 2. All of the atoms in a particular element are identical and of the same type. John Dalton 3. Chemical compounds are made up of atoms joined together. 4. Atoms cannot be created or divided.

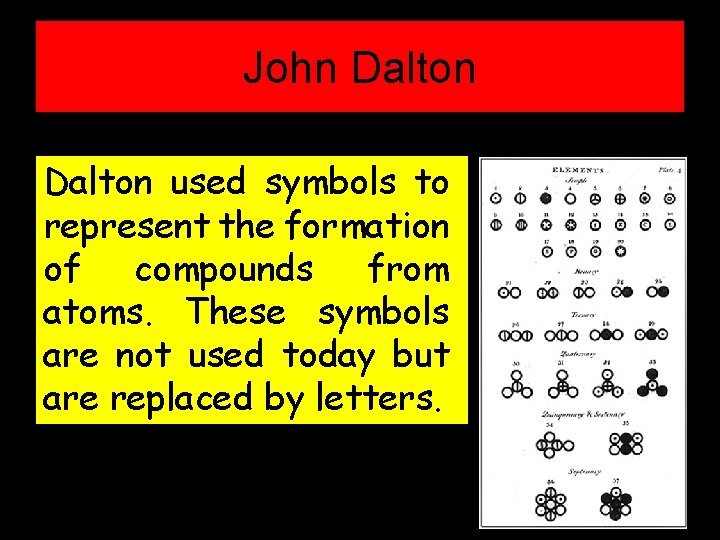
John Dalton used symbols to represent the formation of compounds from atoms. These symbols are not used today but are replaced by letters.

William Crookes In the 1870 s English Scientist, William Crookes, began to study how electricity passed through gases in which there is very low pressure William Crookes


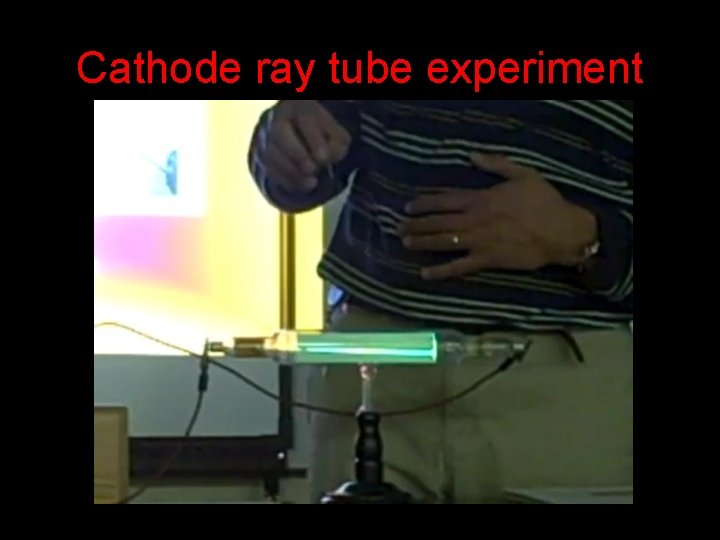
William Crookes Rays coming from the cathode glowed when they struck the glass of the low pressure tube. Discovered that cathode rays : • Travel in straight lines • Small objects placed in the end of the tube cast shadows on the tube http: //ie. youtube. com/watch? v=Xt 7 ZWEDZ_GI

George Johnstone Stoney In 1891, Irish physicist George Johnstone Stoney suggested that cathode rays be termed electrons instead.

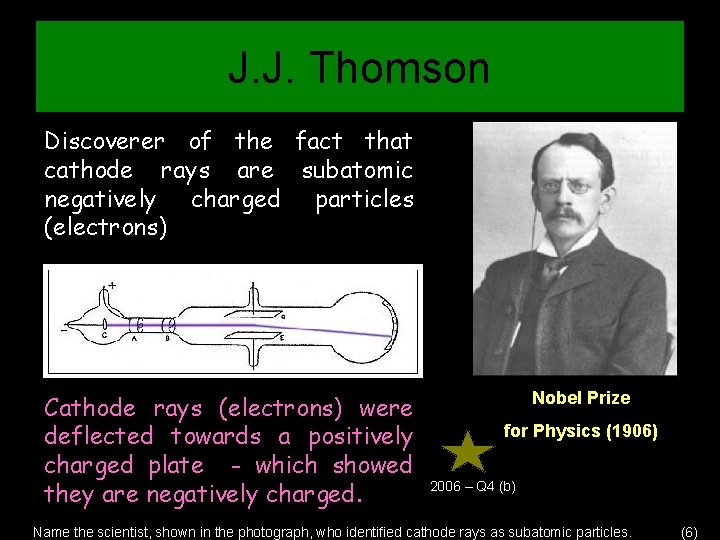
J. J. Thomson Discoverer of the fact that cathode rays are subatomic negatively charged particles (electrons) Cathode rays (electrons) were deflected towards a positively charged plate - which showed they are negatively charged. Nobel Prize for Physics (1906) 2006 – Q 4 (b) Name the scientist, shown in the photograph, who identified cathode rays as subatomic particles. (6)

J. J. Thomson Measured the size of the charge to mass ration (e/m) for cathode ray particles

Cathode ray tube experiment

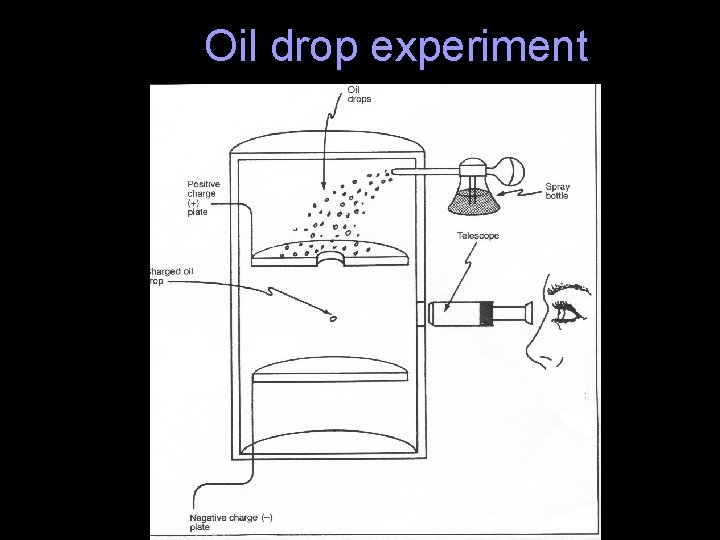
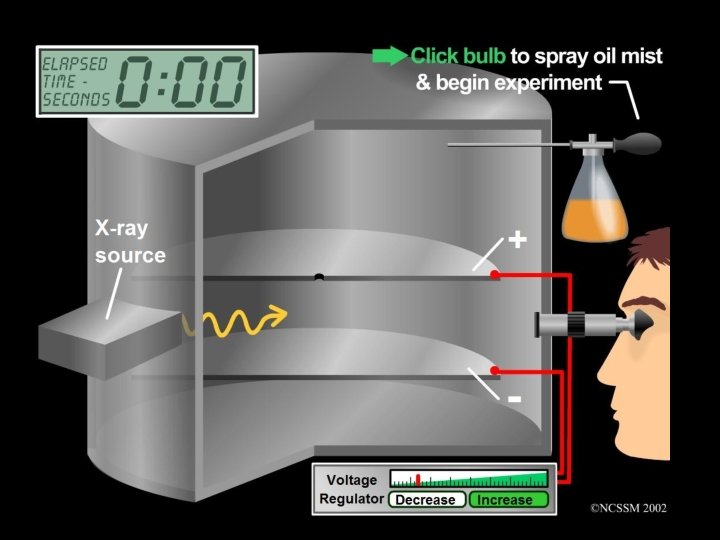
Robert Millikan In 1911 the American Scientist Robert Millikan used his Oil Drop Experiment to measure the size of the charge on the electron. This therefore allowed the mass of the electron to be calculated. Nobel Prize for Physics (1923)

Oil drop experiment


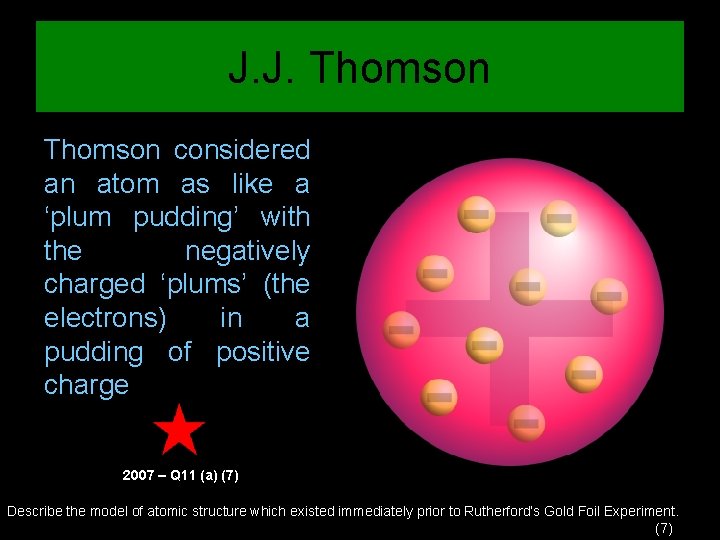
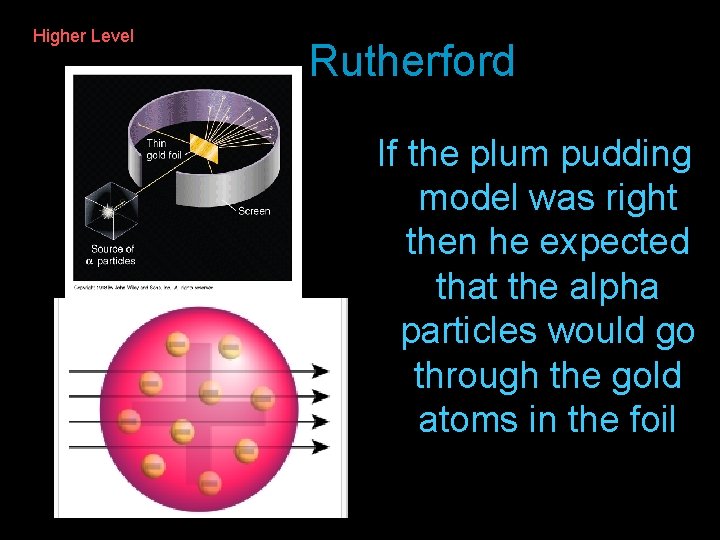
J. J. Thomson considered an atom as like a ‘plum pudding’ with the negatively charged ‘plums’ (the electrons) in a pudding of positive charge 2007 – Q 11 (a) (7) Describe the model of atomic structure which existed immediately prior to Rutherford’s Gold Foil Experiment. (7)

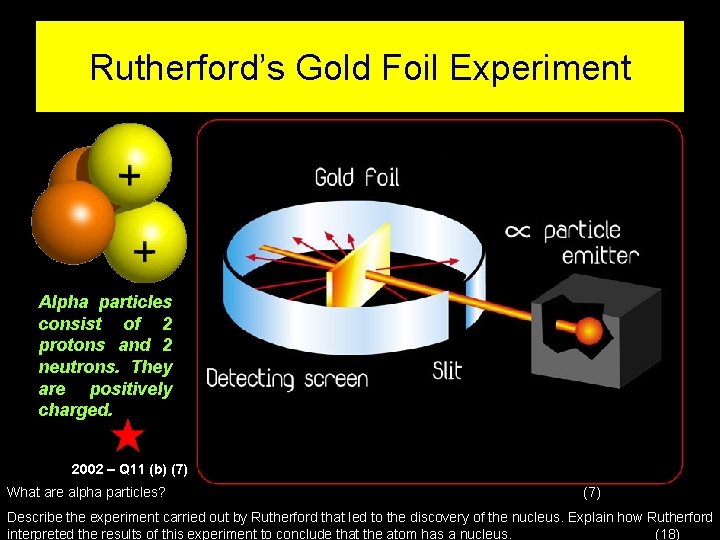
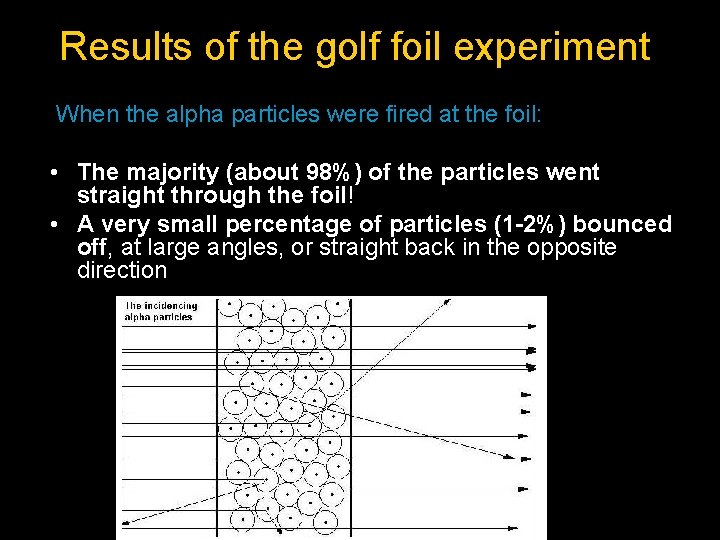
Rutherford’s Gold Foil Experiment Alpha particles consist of 2 protons and 2 neutrons. They are positively charged. 2002 – Q 11 (b) (7) What are alpha particles? (7) Describe the experiment carried out by Rutherford that led to the discovery of the nucleus. Explain how Rutherford interpreted the results of this experiment to conclude that the atom has a nucleus. (18)

Higher Level Rutherford If the plum pudding model was right then he expected that the alpha particles would go through the gold atoms in the foil


Results of the golf foil experiment When the alpha particles were fired at the foil: • The majority (about 98%) of the particles went straight through the foil! • A very small percentage of particles (1 -2%) bounced off, at large angles, or straight back in the opposite direction

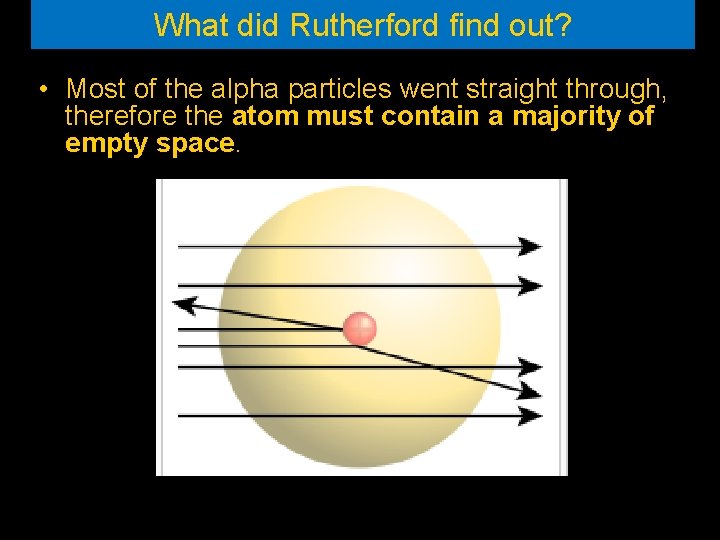
What did Rutherford find out? • Most of the alpha particles went straight through, therefore the atom must contain a majority of empty space.

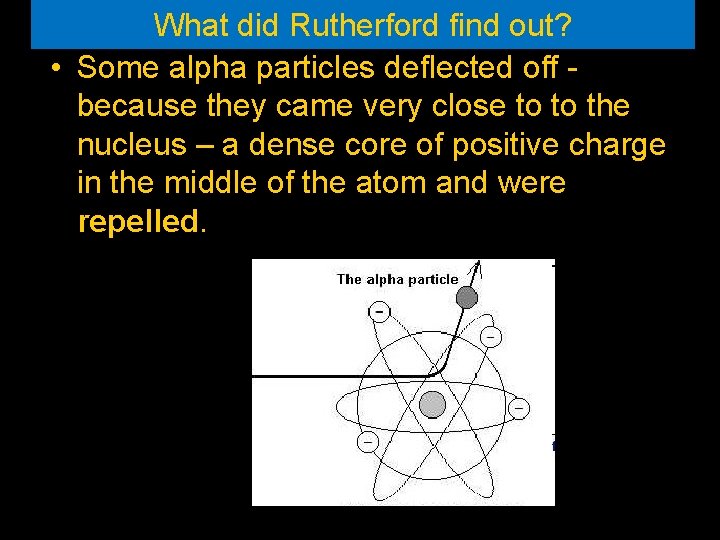
What did Rutherford find out? • Some alpha particles deflected off because they came very close to to the nucleus – a dense core of positive charge in the middle of the atom and were repelled.

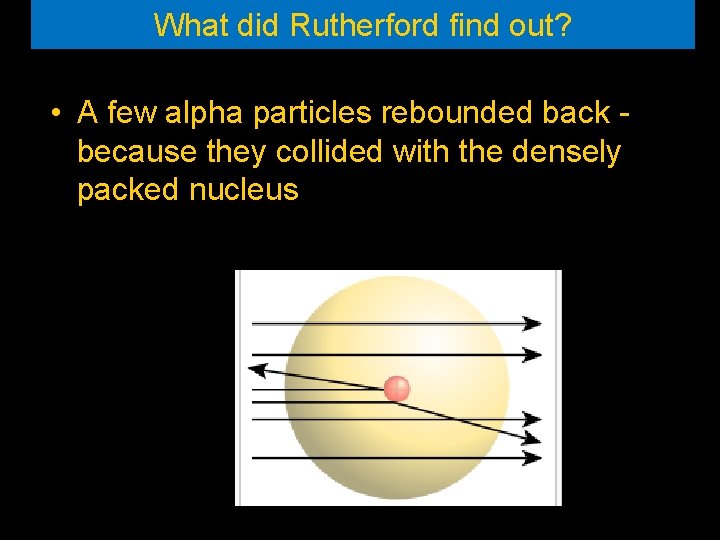
What did Rutherford find out? • A few alpha particles rebounded back because they collided with the densely packed nucleus

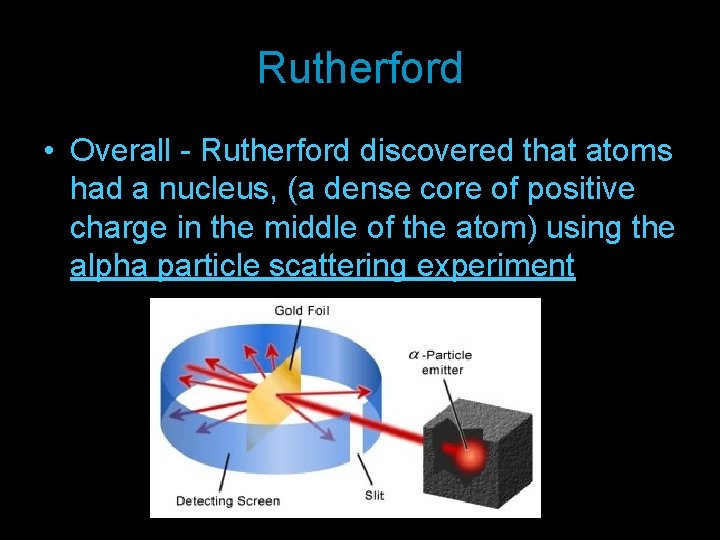
Rutherford • Overall - Rutherford discovered that atoms had a nucleus, (a dense core of positive charge in the middle of the atom) using the alpha particle scattering experiment

Higher Level Rutherford Higher Level • Rutherford called the positive particles in the nucleus “protons” • He discovered protons in the nuclei of various atoms using his alpha particle method.

Bohr Model of the Atom Later, the Danish scientist Niels Bohr, came up with the modern model of the atom which we use today. Nobel Prize for Physics (1922) We will study Bohr’s theories in greater detail in 6 th Year

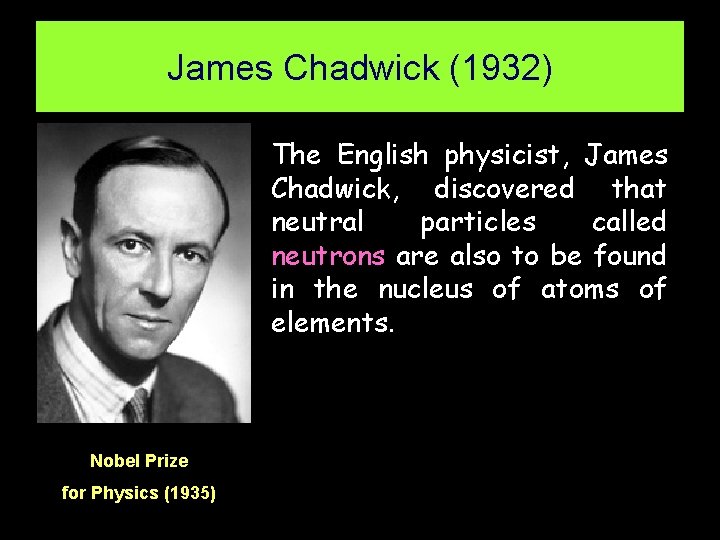
James Chadwick (1932) The English physicist, James Chadwick, discovered that neutral particles called neutrons are also to be found in the nucleus of atoms of elements. Nobel Prize for Physics (1935)

Today’s objectives • • Learning about: Atomic numbers Mass numbers Isotopes

Discovery of atomic structure

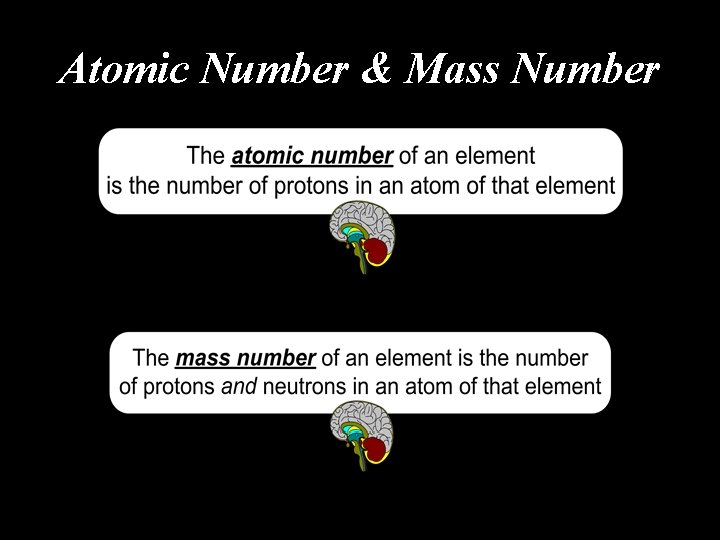
Atomic Number & Mass Number

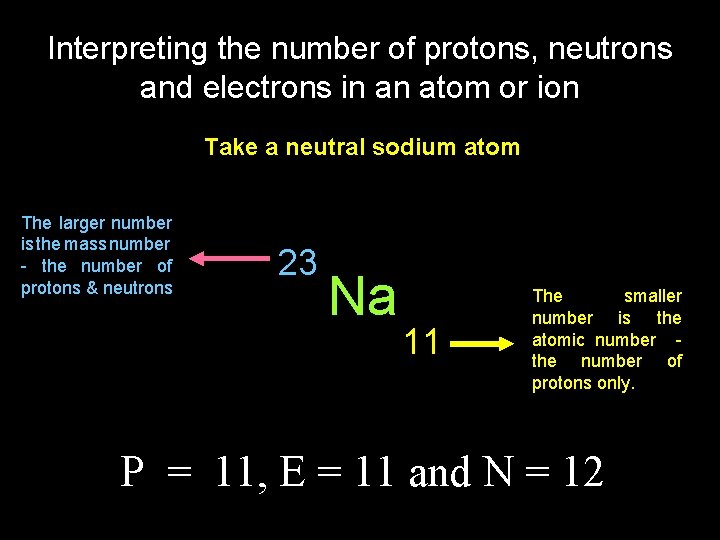
Interpreting the number of protons, neutrons and electrons in an atom or ion Take a neutral sodium atom The larger number is the mass number - the number of protons & neutrons 23 Na 11 The smaller number is the atomic number the number of protons only. P = 11, E = 11 and N = 12

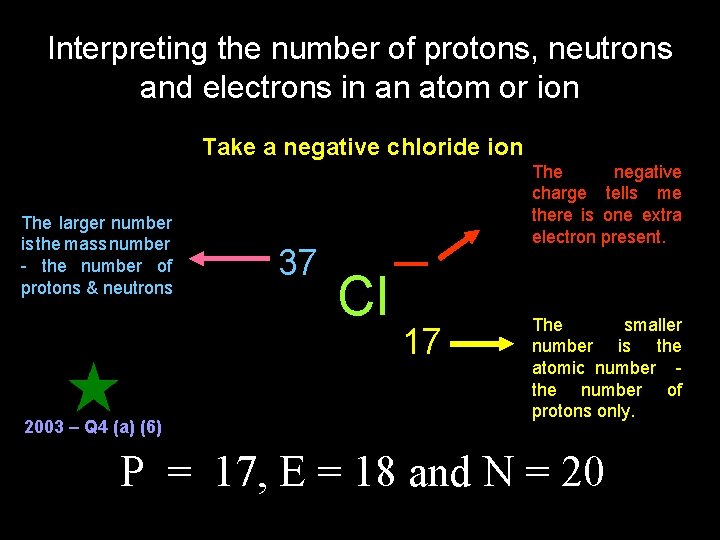
Interpreting the number of protons, neutrons and electrons in an atom or ion Take a negative chloride ion The larger number is the mass number - the number of protons & neutrons 2003 – Q 4 (a) (6) 37 The negative charge tells me there is one extra electron present. Cl 17 The smaller number is the atomic number the number of protons only. P = 17, E = 18 and N = 20

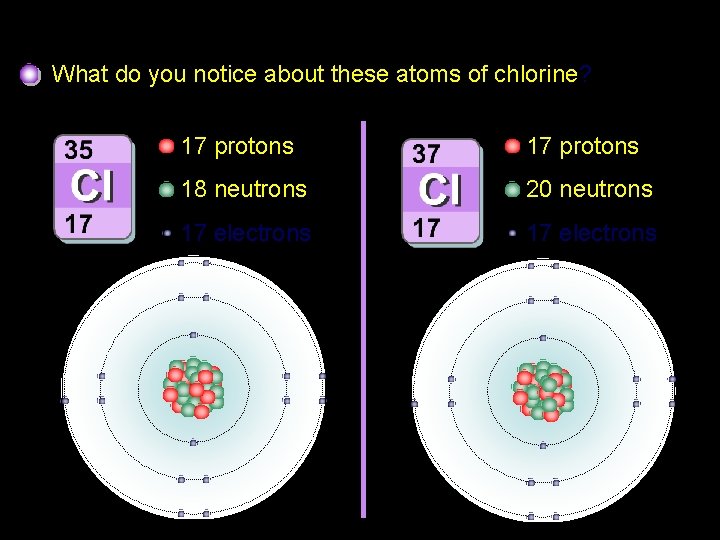
Isotopes of chlorine What do you notice about these atoms of chlorine? 17 protons 18 neutrons 20 neutrons 17 electrons

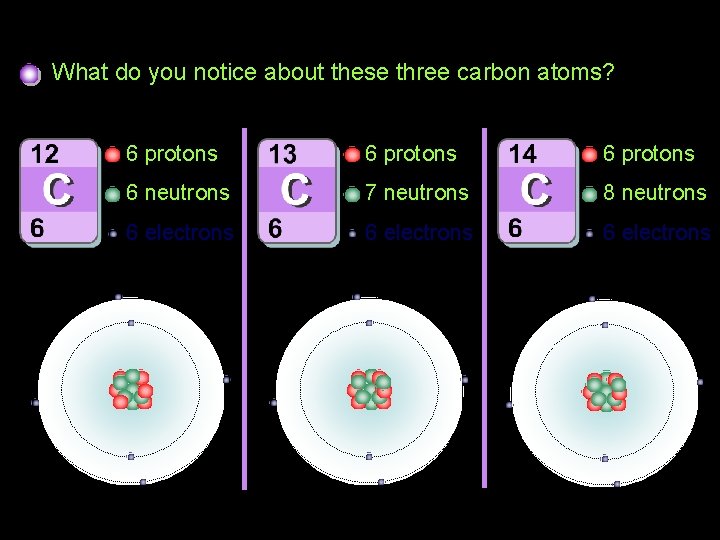
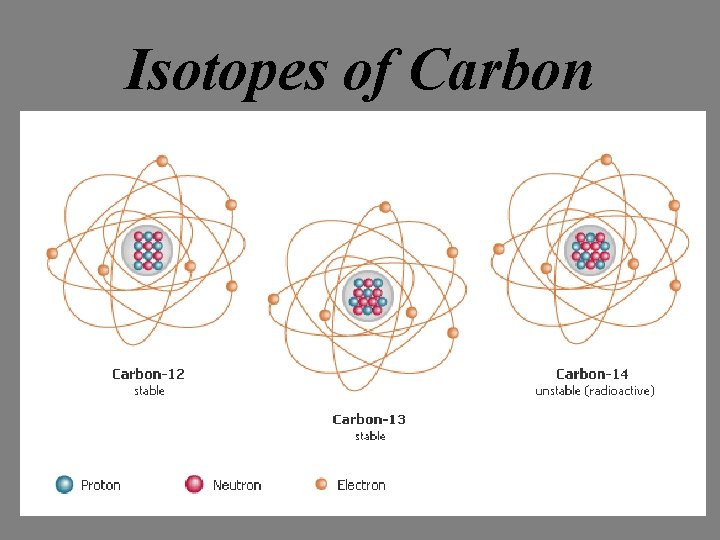
Isotopes of carbon What do you notice about these three carbon atoms? 6 protons 6 neutrons 7 neutrons 8 neutrons 6 electrons

Isotopes

Isotopes of Carbon

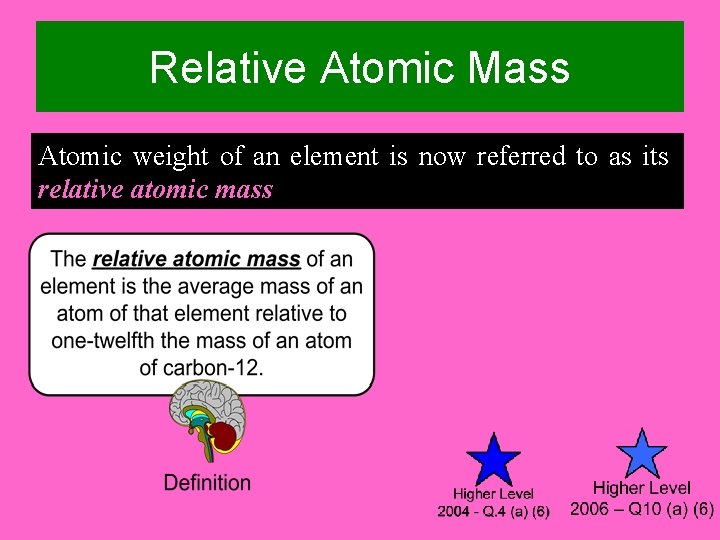
Relative Atomic Mass Atomic weight of an element is now referred to as its relative atomic mass

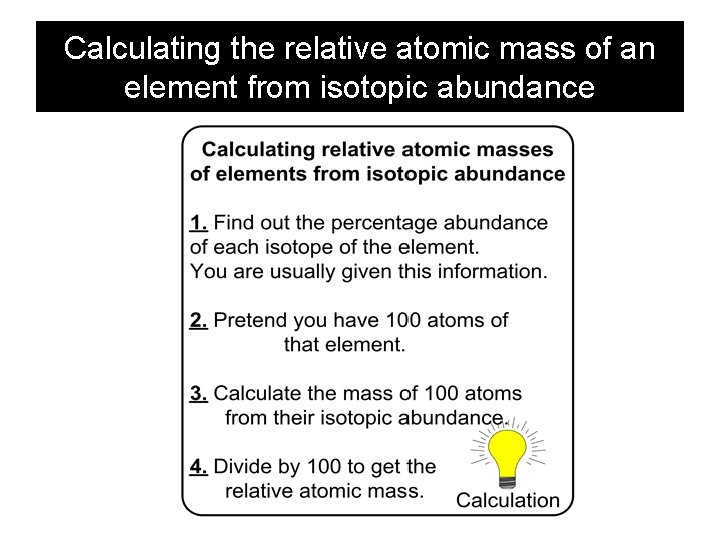
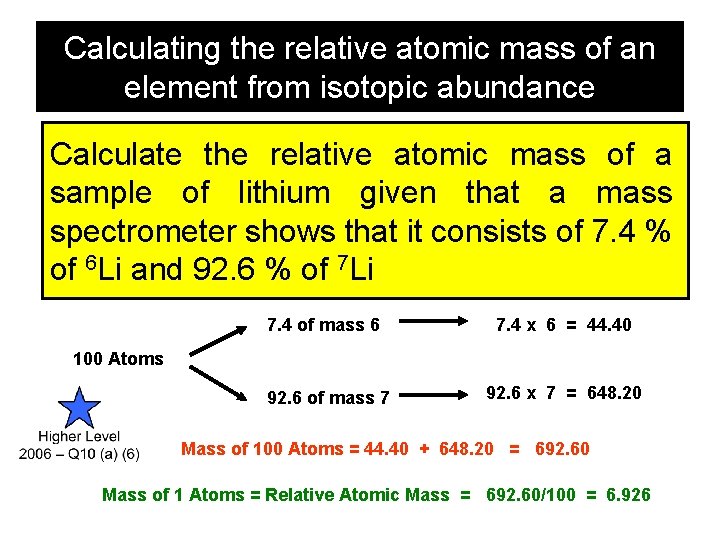
Calculating the relative atomic mass of an element from isotopic abundance

Calculating the relative atomic mass of an element from isotopic abundance Calculate the relative atomic mass of a sample of lithium given that a mass spectrometer shows that it consists of 7. 4 % of 6 Li and 92. 6 % of 7 Li 7. 4 of mass 6 7. 4 x 6 = 44. 40 100 Atoms 92. 6 of mass 7 92. 6 x 7 = 648. 20 Mass of 100 Atoms = 44. 40 + 648. 20 = 692. 60 Mass of 1 Atoms = Relative Atomic Mass = 692. 60/100 = 6. 926

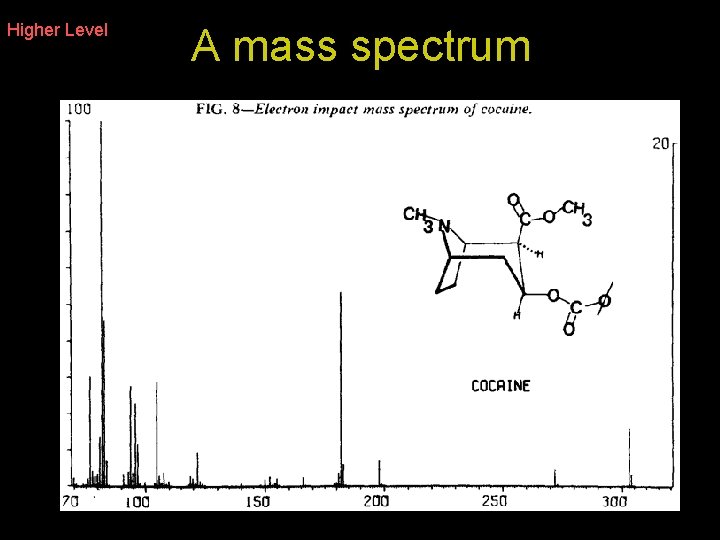
Mass spectrometer in determining relative atomic masses The mass spectrometer can be used to measure relative atomic masses. It is also commonly used to determine concentrations of drugs in urine samples

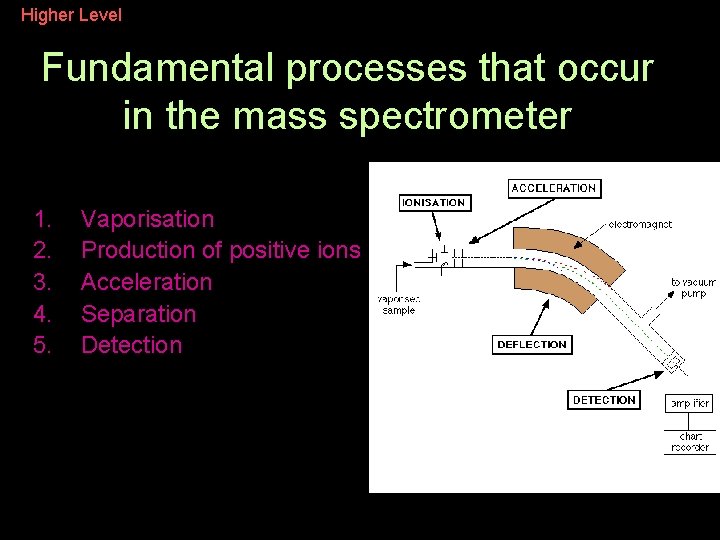
Higher Level Fundamental processes that occur in the mass spectrometer 1. 2. 3. 4. 5. Vaporisation Production of positive ions Acceleration Separation Detection

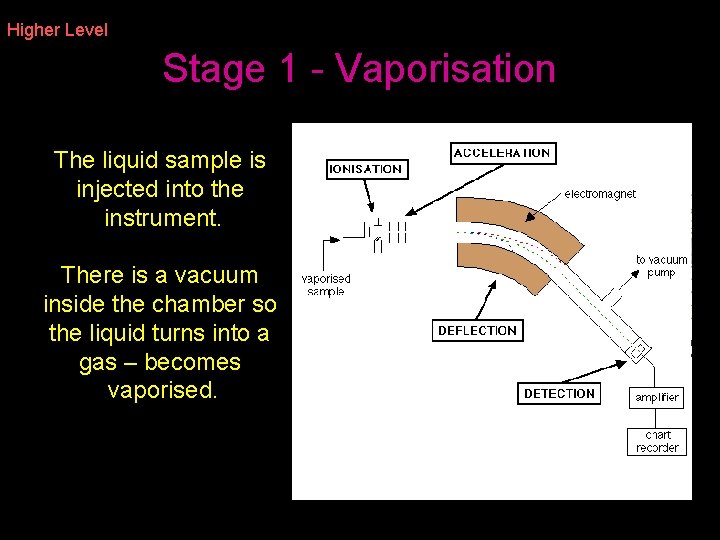
Higher Level Stage 1 - Vaporisation The liquid sample is injected into the instrument. There is a vacuum inside the chamber so the liquid turns into a gas – becomes vaporised.

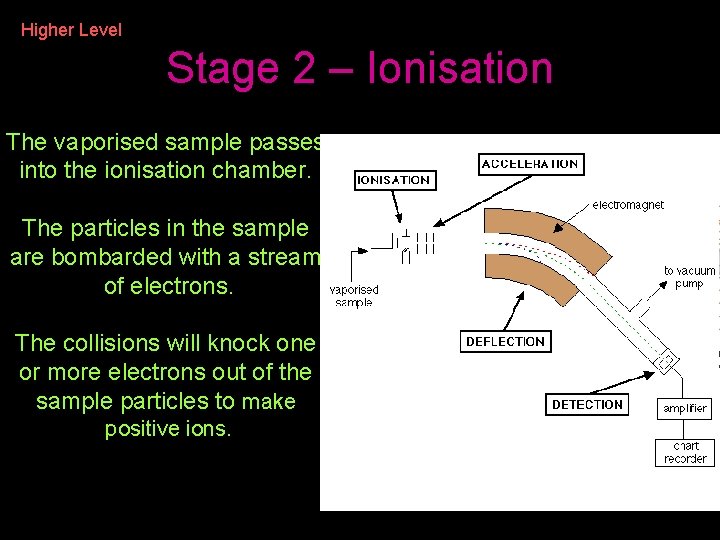
Higher Level Stage 2 – Ionisation The vaporised sample passes into the ionisation chamber. The particles in the sample are bombarded with a stream of electrons. The collisions will knock one or more electrons out of the sample particles to make positive ions.

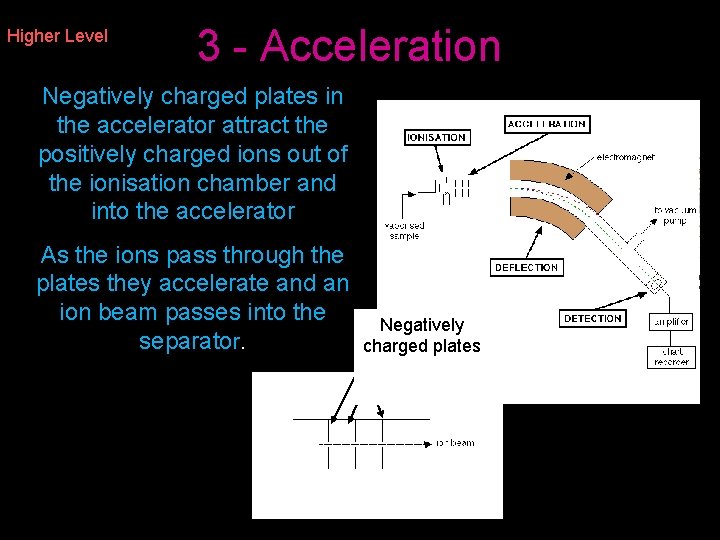
Higher Level 3 - Acceleration Negatively charged plates in the accelerator attract the positively charged ions out of the ionisation chamber and into the accelerator As the ions pass through the plates they accelerate and an ion beam passes into the separator. Negatively charged plates

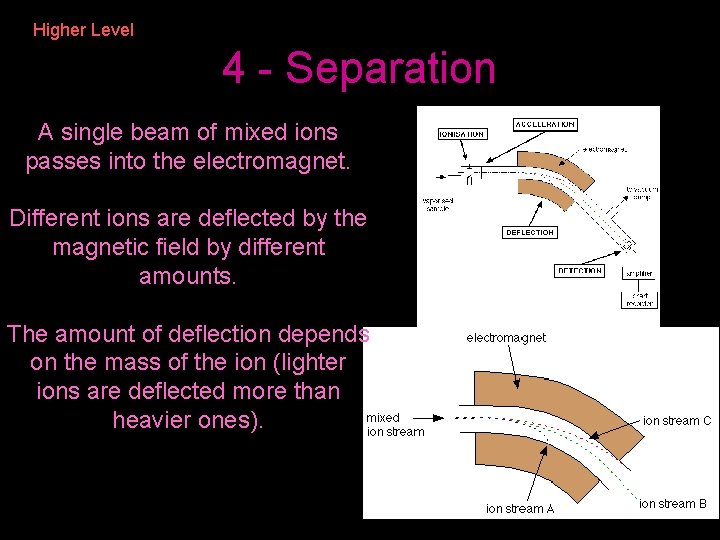
Higher Level 4 - Separation A single beam of mixed ions passes into the electromagnet. Different ions are deflected by the magnetic field by different amounts. The amount of deflection depends on the mass of the ion (lighter ions are deflected more than heavier ones).

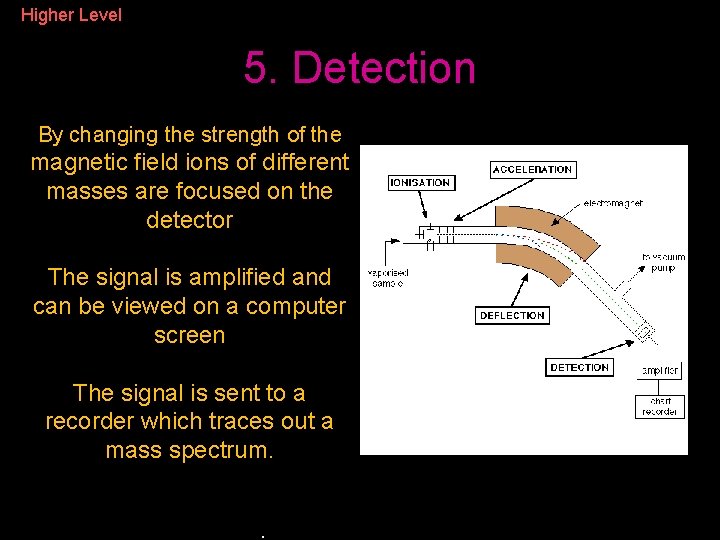
Higher Level 5. Detection By changing the strength of the magnetic field ions of different masses are focused on the detector The signal is amplified and can be viewed on a computer screen The signal is sent to a recorder which traces out a mass spectrum. The mass spectrum is interpreted by the scientist.

Higher Level A mass spectrum

Mass spec video – Go to 3 mins 0 4 mins