2 8 Naming Inorganic Compounds AP Chemistry Summer












- Slides: 18

2. 8 – Naming Inorganic Compounds AP Chemistry Summer Homework Chapter 2

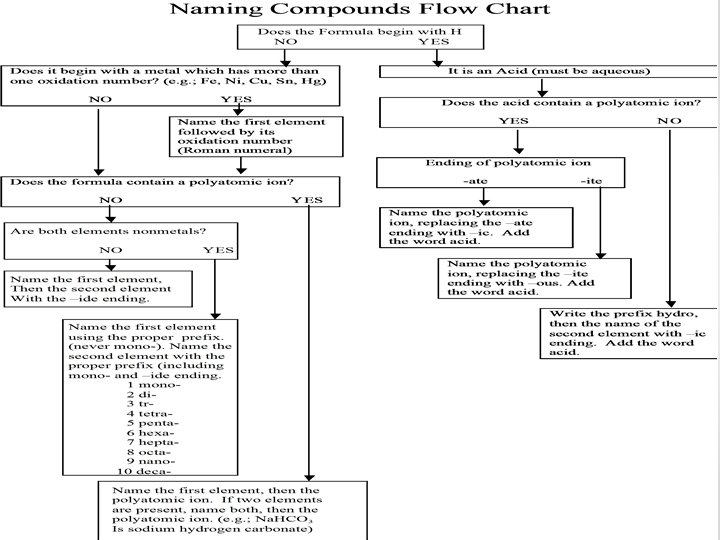
Naming • There almost an infinite number of ways in which element can combine in order to make compounds • Therefore, a systematic method is used so that we can easily name all compounds • We will focus on naming inorganic (all non-carbon containing) compounds in this lecture

Names and Formulas of Ionic Compounds: Cations 1. Cations formed from metal atoms have the same name as the metal o E. g. : Na+ is a sodium ion, Zn+2 is a zinc ion, etc. 2. If a metal can have multiple charges, the positive charge is indicated by a Roman numeral in parentheses following the name of the metal. o E. g. : Fe 2+ = iron (II) ion, Cu+3 = copper (III) ion o Iron, cobalt, copper, tin, and lead are the most common metals that have multiple charges

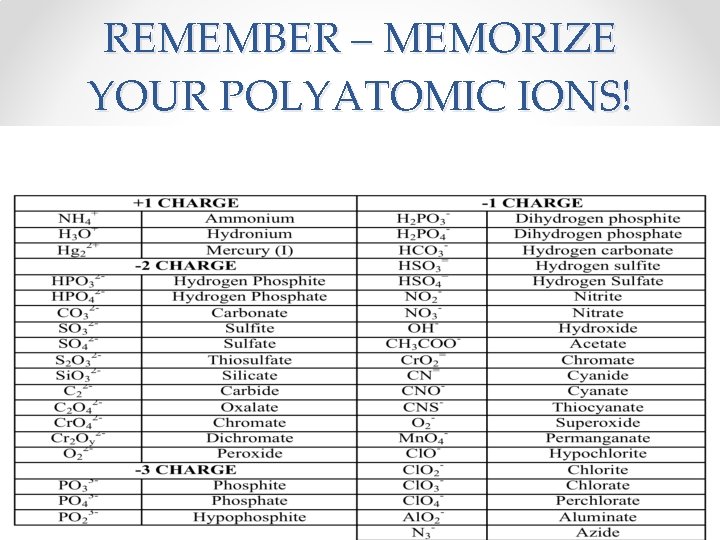
REMEMBER – MEMORIZE YOUR POLYATOMIC IONS!

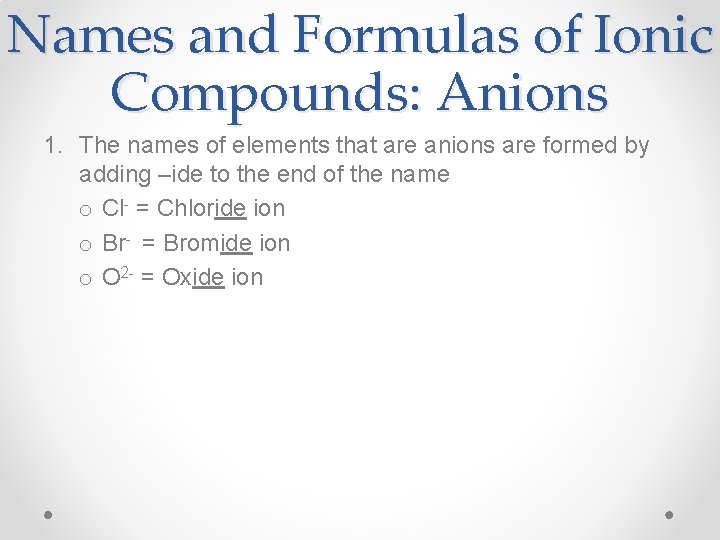
Names and Formulas of Ionic Compounds: Anions 1. The names of elements that are anions are formed by adding –ide to the end of the name o Cl- = Chloride ion o Br- = Bromide ion o O 2 - = Oxide ion

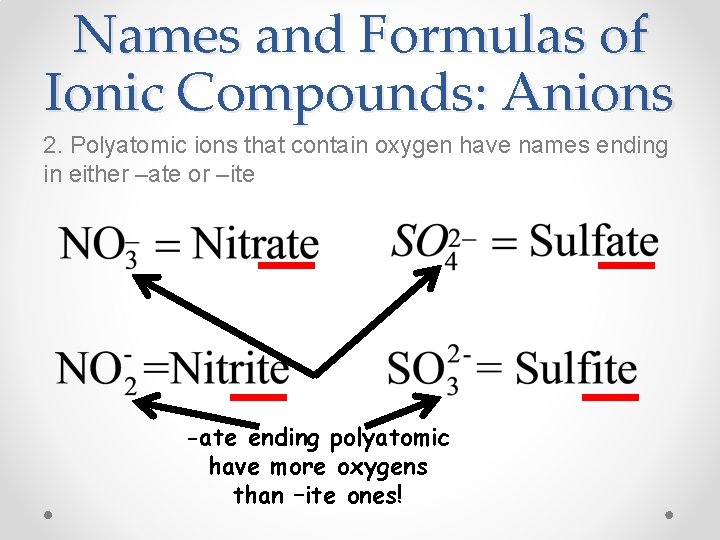
Names and Formulas of Ionic Compounds: Anions 2. Polyatomic ions that contain oxygen have names ending in either –ate or –ite -ate ending polyatomic have more oxygens than –ite ones!

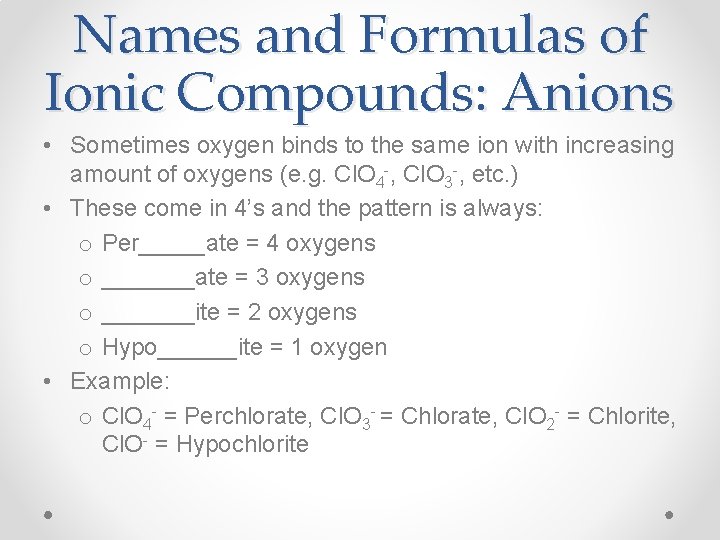
Names and Formulas of Ionic Compounds: Anions • Sometimes oxygen binds to the same ion with increasing amount of oxygens (e. g. Cl. O 4 -, Cl. O 3 -, etc. ) • These come in 4’s and the pattern is always: o Per_____ate = 4 oxygens o _______ate = 3 oxygens o _______ite = 2 oxygens o Hypo______ite = 1 oxygen • Example: o Cl. O 4 - = Perchlorate, Cl. O 3 - = Chlorate, Cl. O 2 - = Chlorite, Cl. O- = Hypochlorite

Names and Formulas of Ionic Compounds: Anions 3. Anions derived by adding H+ to an oxygen containing anions have the prefix of hydrogen or dihydrogen based on the number of H+ in the anion

Naming Ionic Compounds • To name ionic compounds, you always name the cation and then the anion.

Class Example Name the following compounds: 1. Ca. Cl 2 2. Ba(OH)2 3. Sodium hydroxide

Try It Out! 1. K 2 SO 4 2. Fe. Cl 3 3. Cobalt (II) Nitrate

Names and Formulas for Acids • Recall, acids are compounds that donate a hydrogen ion to solution. • Acids are named differently than other compounds • Two rules when naming acids: 1. Acids containing anions that are only single elements are named by changing the –ide prefix on the anion to –ic and adding hydro in front of the anion and then adding acid at the end. • E. g. HCl is hydrochloric acid 2. Acids containing polyatomic ions are named by changing –ate to –ic and –ite to –ous on the anion and then adding acid at the end

Class Example 1. HBr 2. H 2 SO 4 3. HCl. O 2

Try It Out! 1. HNO 3 2. HF

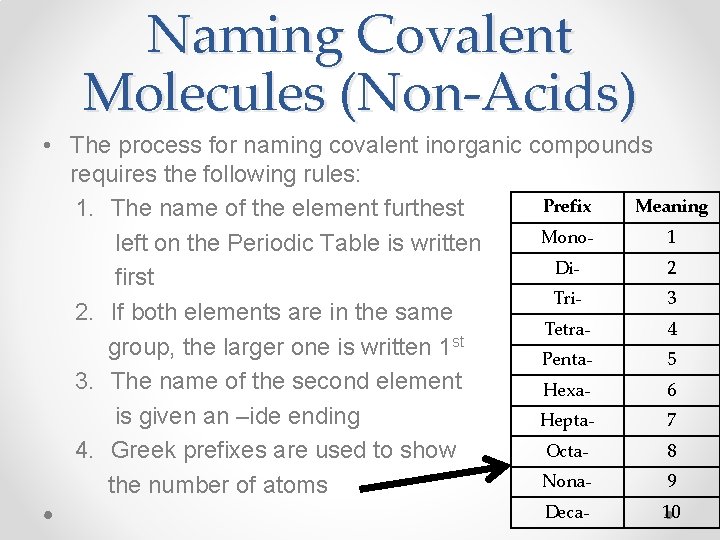
Naming Covalent Molecules (Non-Acids) • The process for naming covalent inorganic compounds requires the following rules: Prefix Meaning 1. The name of the element furthest Mono 1 left on the Periodic Table is written Di 2 first Tri 3 2. If both elements are in the same Tetra 4 st group, the larger one is written 1 Penta 5 3. The name of the second element Hexa 6 is given an –ide ending Hepta 7 Octa 8 4. Greek prefixes are used to show Nona 9 the number of atoms Deca- 10

Class Example 1. Si. Br 4 2. Disulfur dichloride 3. NH 3

Try It Out! 1. N 2 O 2. Tetraphosphorus hexasulfide
