2 4 Names and Formulae of Inorganic Compounds





















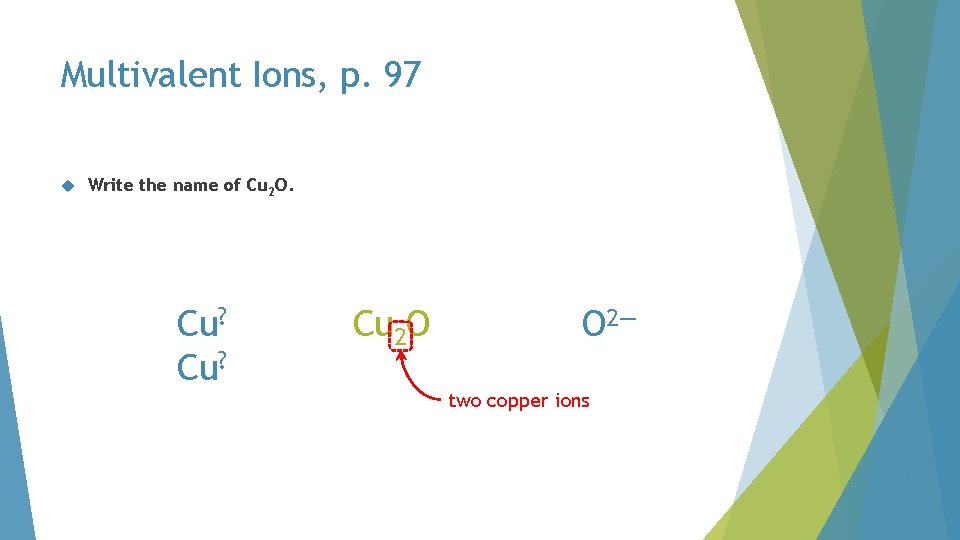
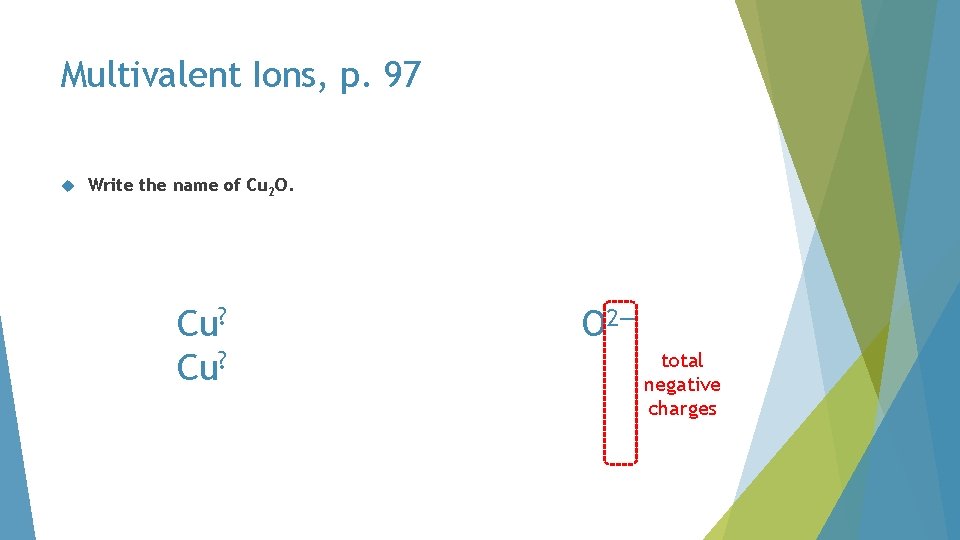




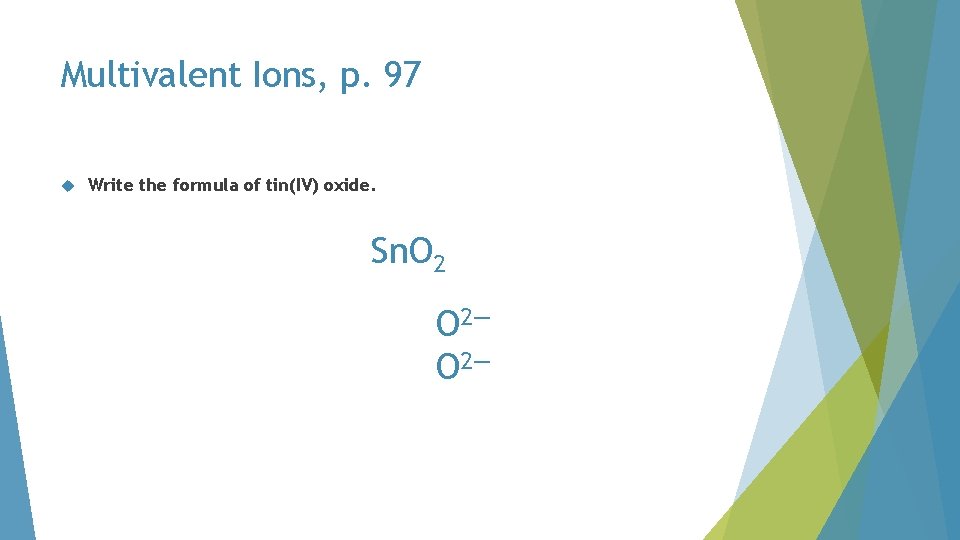












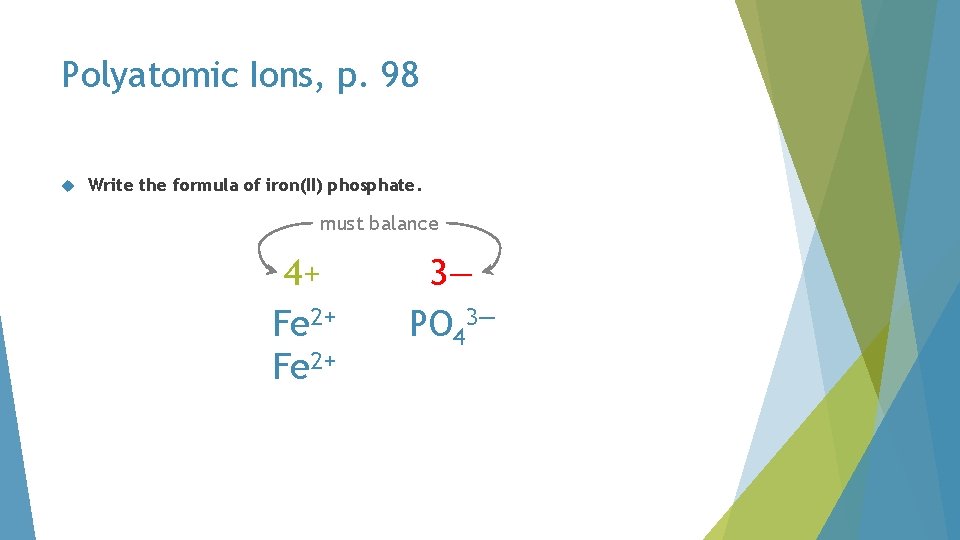
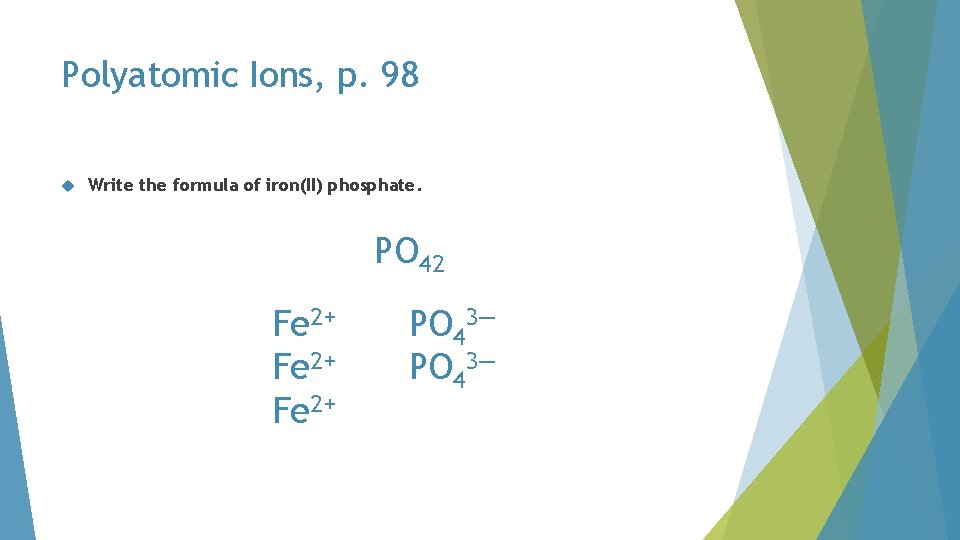
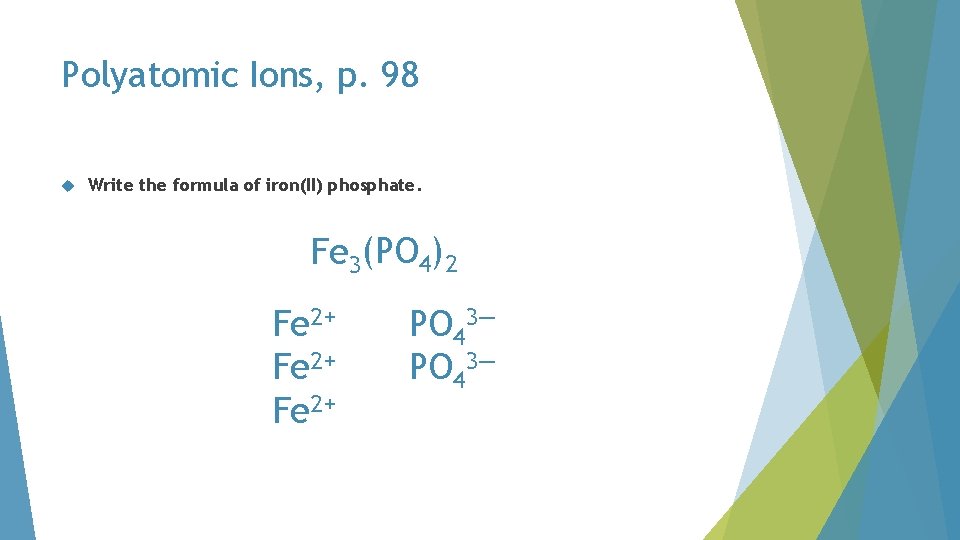



- Slides: 89

2. 4 – Names and Formulae of Inorganic Compounds

Binary Ionic Compounds, p. 96 You can recognize the formula of a binary compound because it only contains two capital letters, one for each elemental symbol

Binary Ionic Compounds, p. 96 Monoatomic ion Any ion derived from a single atom one + atom monoatomic

Binary Ionic Compounds, p. 96 Binary ionic compounds contain one type of monoatomic metal cation and one type of monoatomic non-metal anion

Multivalent Ions, p. 97 Multivalent metal Any metal that has multiple possible ionic charges

Multivalent Ions, p. 97 The name of every ionic compound starts with the name of its cation and ends with the name of its anion

Na + Na

sodium Na + Na

sodium Na + sodium Na ion

3+ Ti 4+ Ti

3+ titanium(III) Ti 4+ Ti ion

3+ titanium(III) Ti ion 4+ titanium(IV) Ti ion

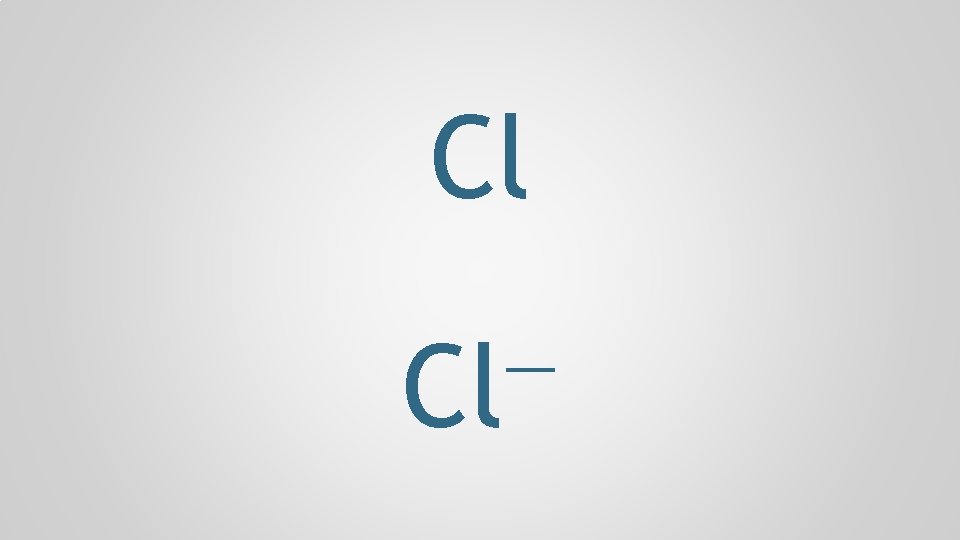
Cl — Cl

chlorine Cl — Cl

chlorine Cl — chloride Cl

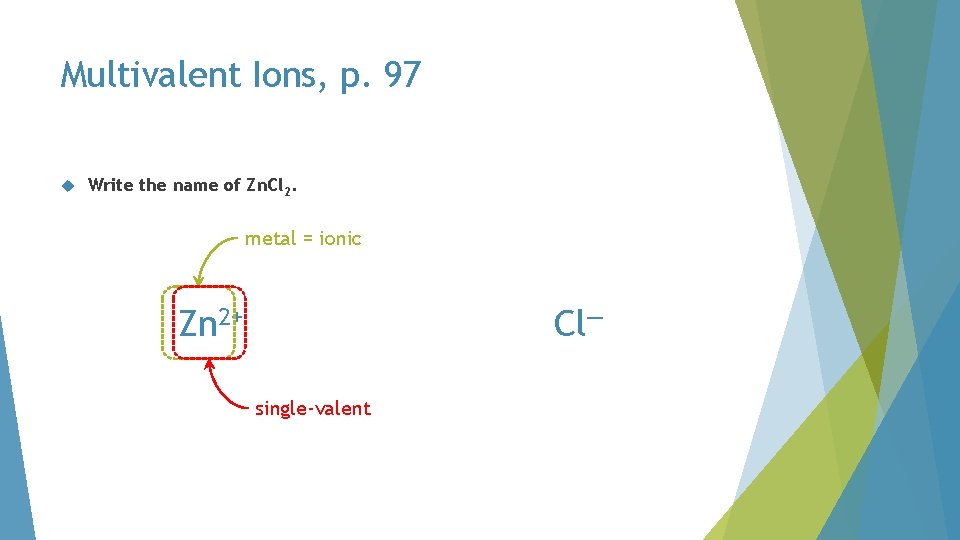
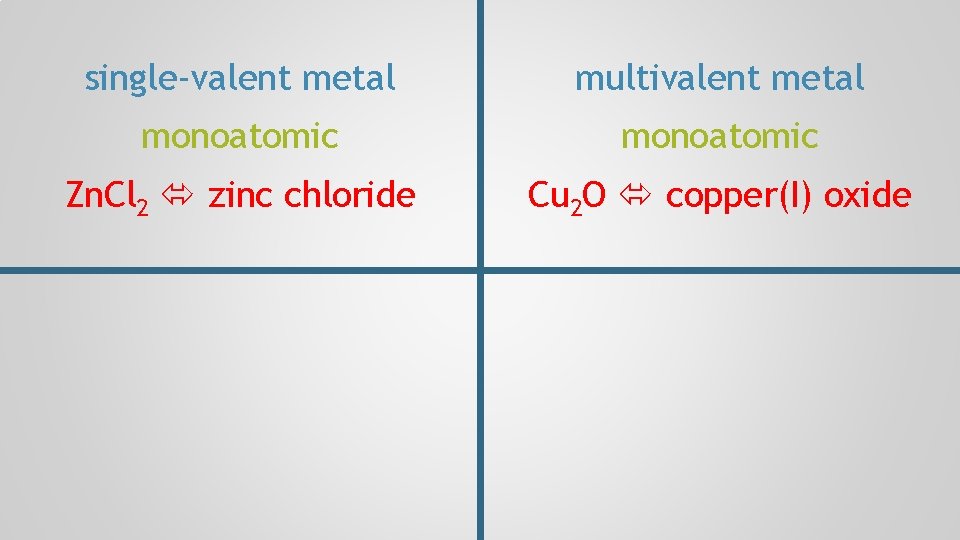
Multivalent Ions, p. 97 Write the name of Zn. Cl 2

Multivalent Ions, p. 97 Write the name of Zn. Cl 2. Zn. Cl

Multivalent Ions, p. 97 Write the name of Zn. Cl 2. metal = ionic Cl— Zn 2+ single-valent

Multivalent Ions, p. 97 Write the name of Zn. Cl 2. zinc chloride Cl—

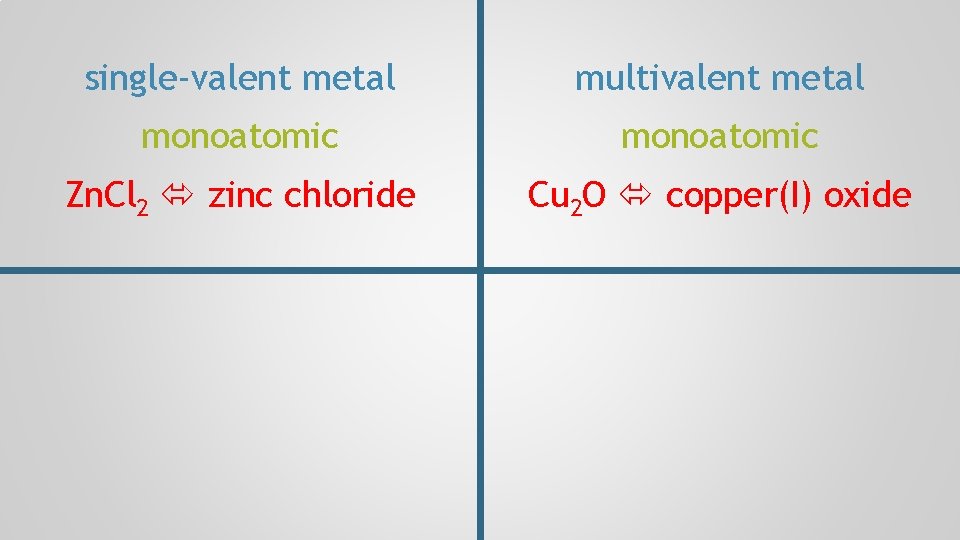
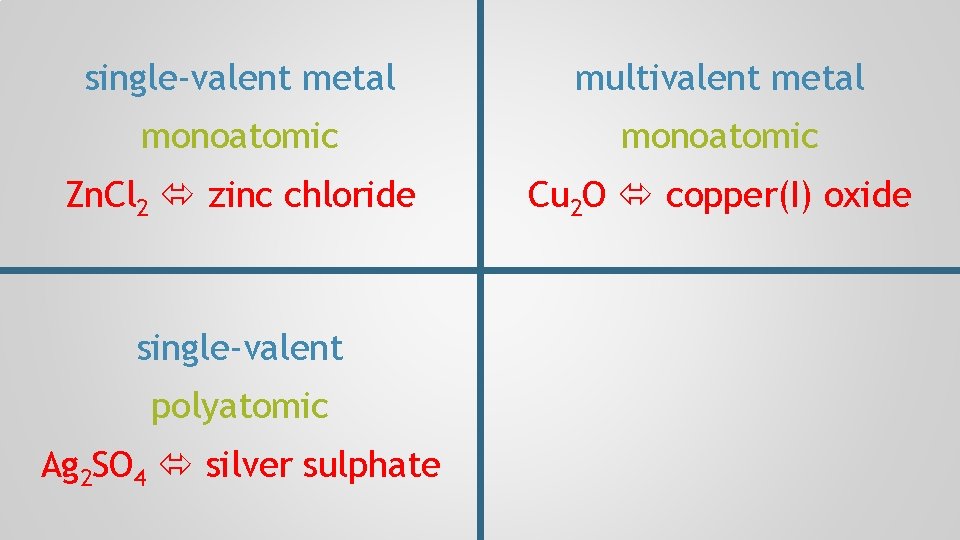
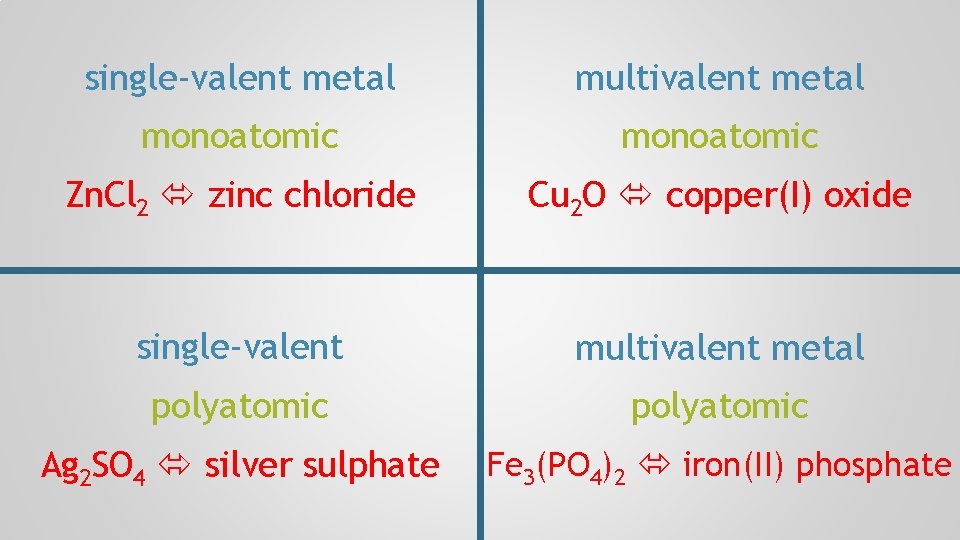
single-valent metal monoatomic Zn. Cl 2 zinc chloride

Multivalent Ions, p. 97 Just as all atoms are neutral, despite being made up of charged particles, all compounds are neutral, requiring that every positive cation charge have a corresponding negative anion charge

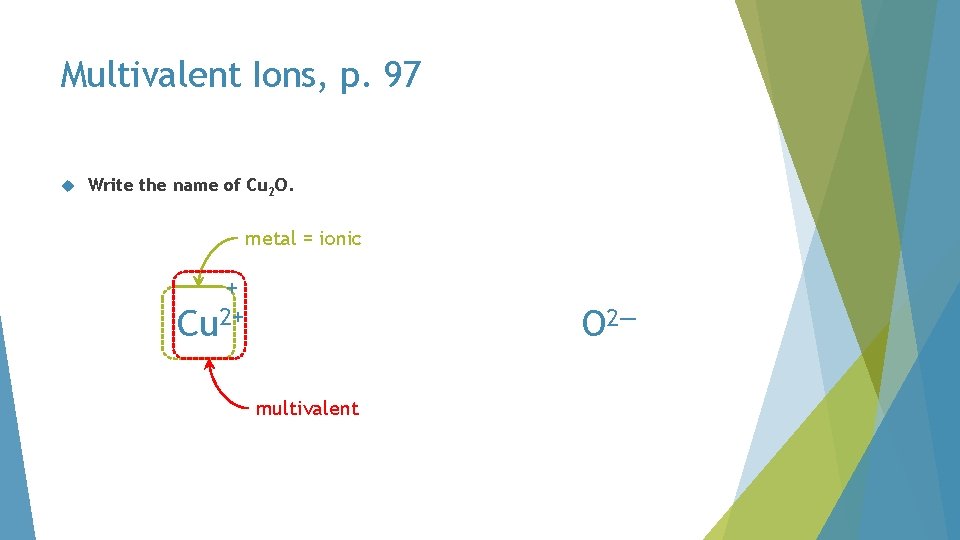
Multivalent Ions, p. 97 Write the name of Cu 2 O

Multivalent Ions, p. 97 Write the name of Cu 2 O. Cu O

Multivalent Ions, p. 97 Write the name of Cu 2 O. metal = ionic + Cu 2+ O 2— multivalent
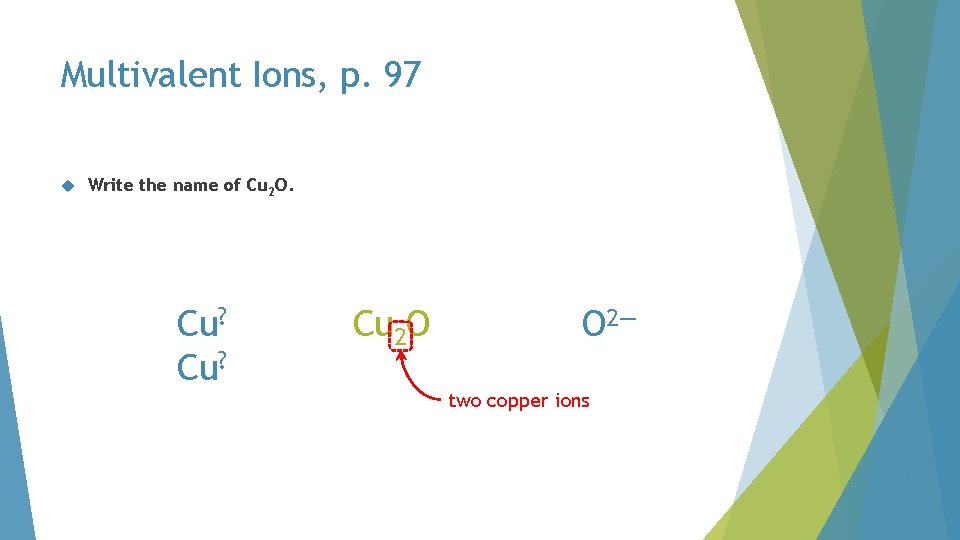
Multivalent Ions, p. 97 Write the name of Cu 2 O. Cu? Cu 2 O O 2— two copper ions
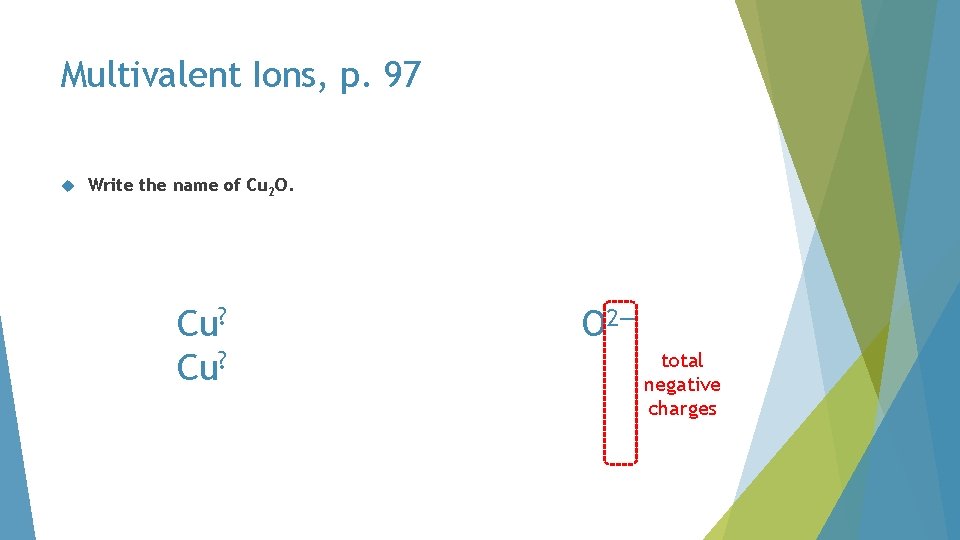
Multivalent Ions, p. 97 Write the name of Cu 2 O. Cu? O 2— total negative charges

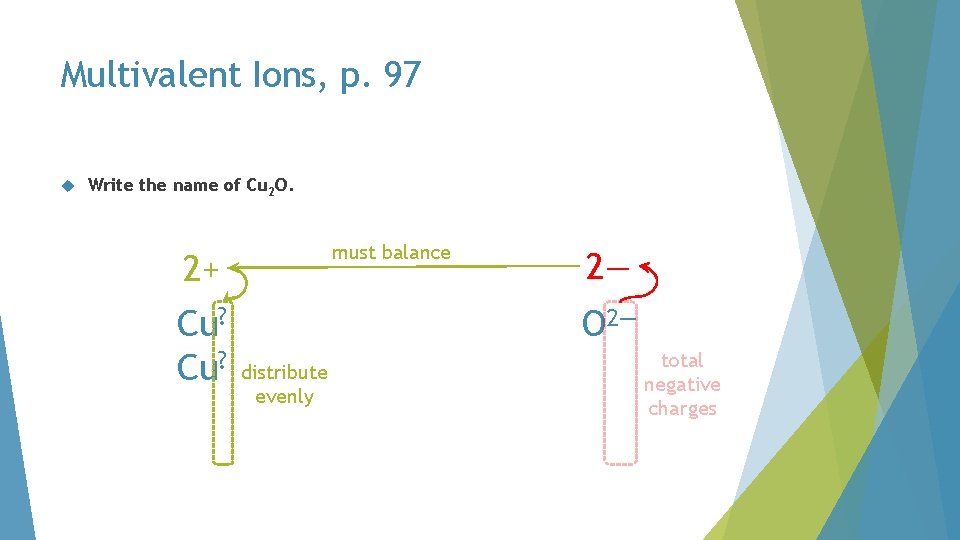
Multivalent Ions, p. 97 Write the name of Cu 2 O. must balance 2+ Cu? 2— O 2— distribute evenly total negative charges

Multivalent Ions, p. 97 Write the name of Cu 2 O. Cu+ copper(I) oxide O 2—

single-valent metal multivalent metal monoatomic Zn. Cl 2 zinc chloride Cu 2 O copper(I) oxide

Multivalent Ions, p. 97 To determine an ionic compound’s formula from its name, you must determine the simplest ratio between the cation and the anion that successfully balances the positive and negative charges

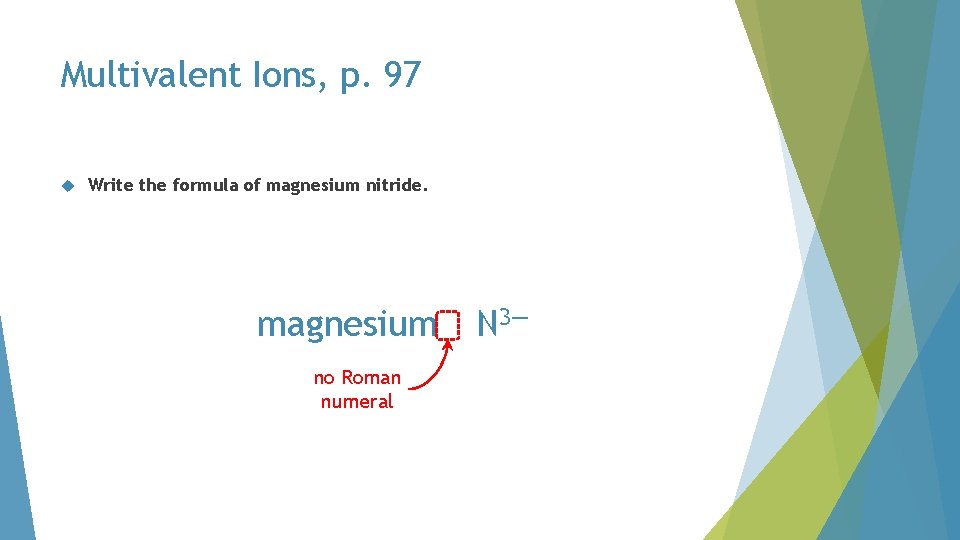
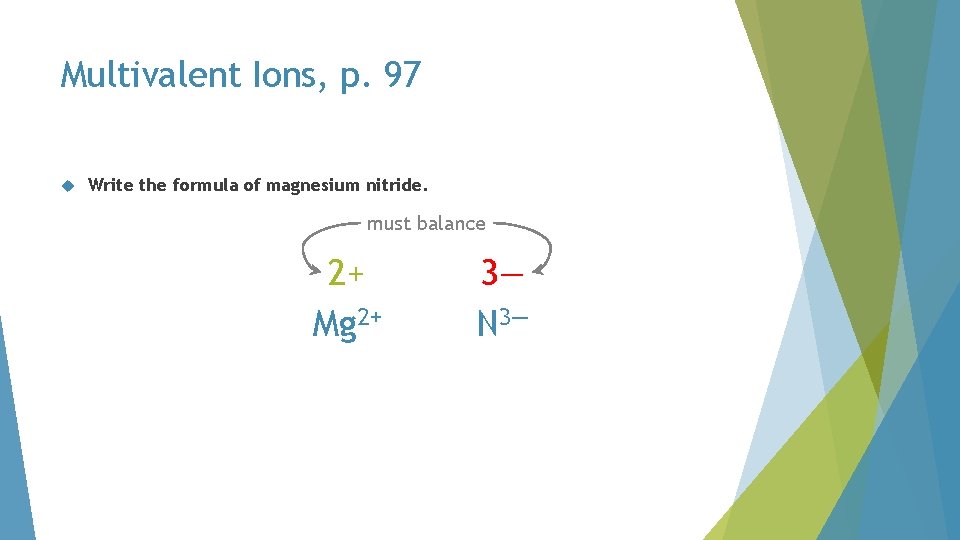
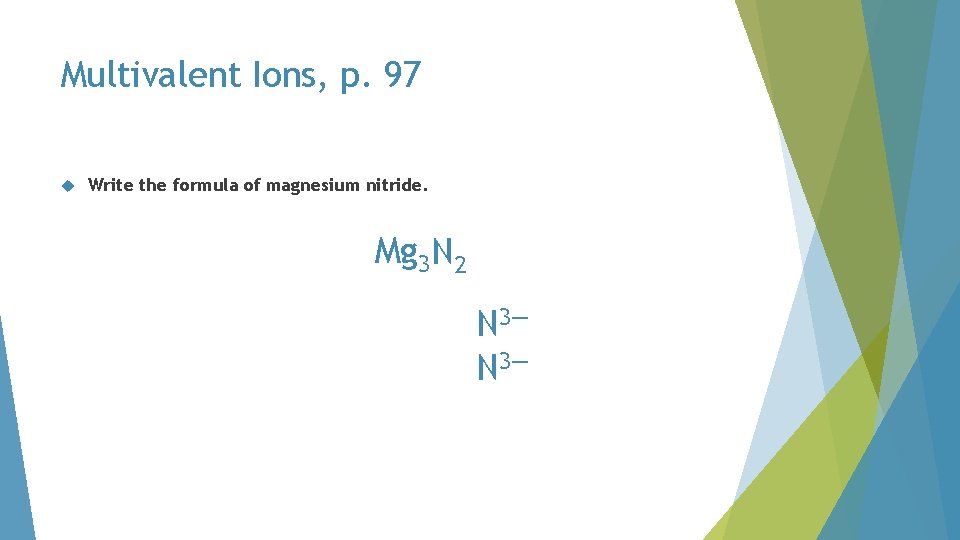
Multivalent Ions, p. 97 Write the formula of magnesium nitride

Multivalent Ions, p. 97 Write the formula of magnesium nitride. magnesium no Roman numeral N 3—

Multivalent Ions, p. 97 Write the formula of magnesium nitride. must balance 2+ Mg 2+ 3— N 3—

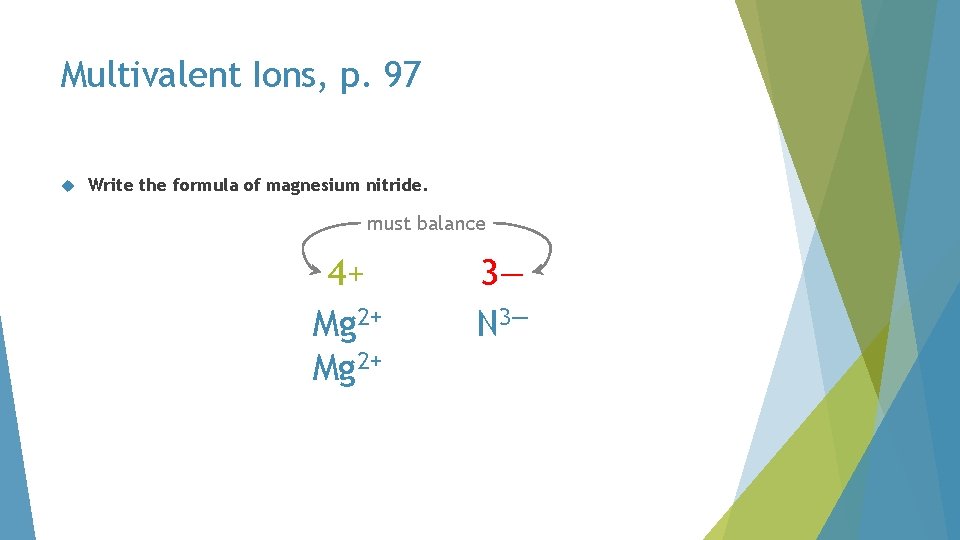
Multivalent Ions, p. 97 Write the formula of magnesium nitride. must balance 4+ Mg 2+ 3— N 3—

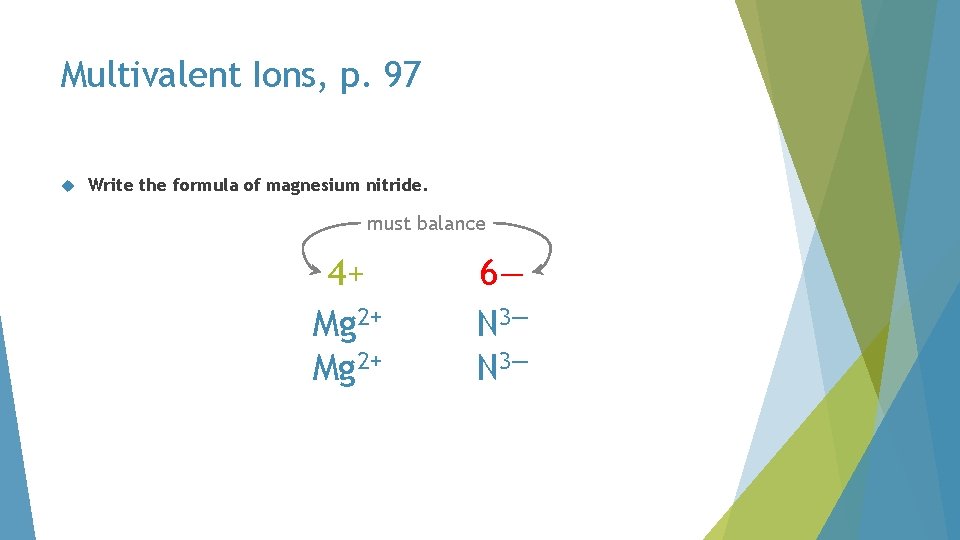
Multivalent Ions, p. 97 Write the formula of magnesium nitride. must balance 4+ Mg 2+ 6— N 3—

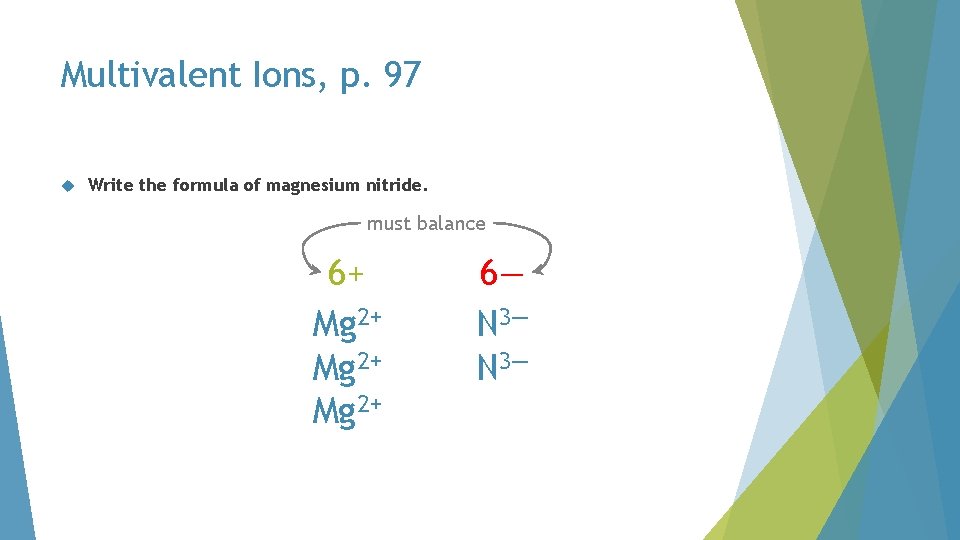
Multivalent Ions, p. 97 Write the formula of magnesium nitride. must balance 6+ Mg 2+ 6— N 3—

Multivalent Ions, p. 97 Write the formula of magnesium nitride. Mg 3 N 2 N 3—

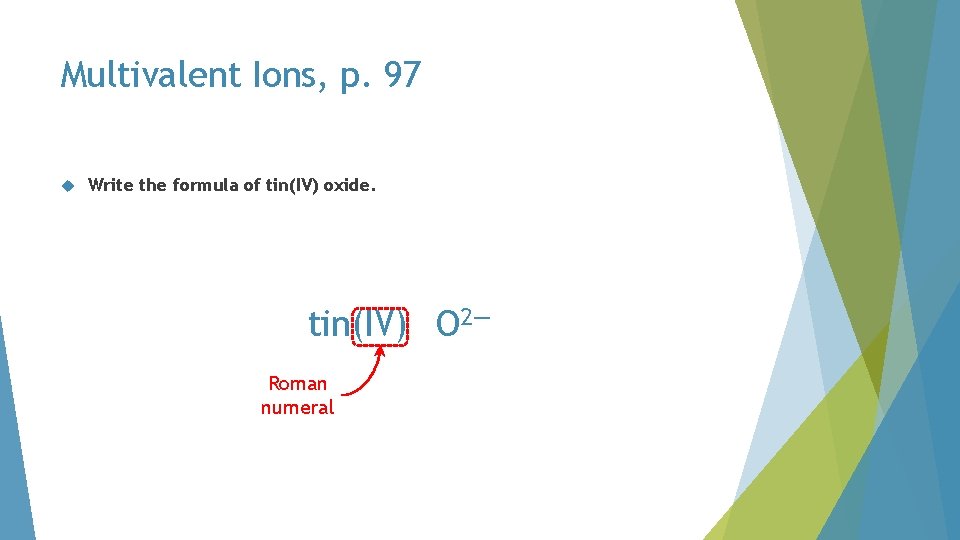
Multivalent Ions, p. 97 Write the formula of tin(IV) oxide

Multivalent Ions, p. 97 Write the formula of tin(IV) oxide. tin(IV) O 2— Roman numeral

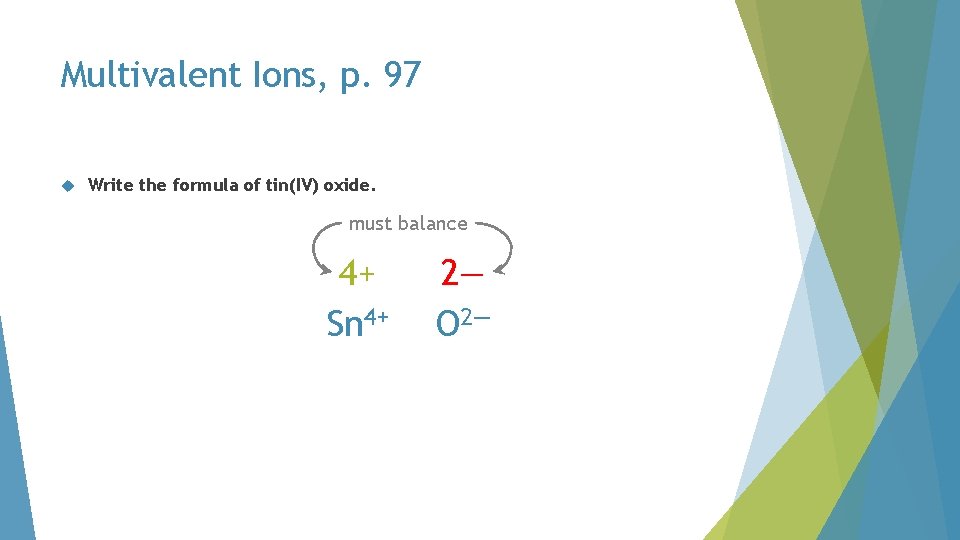
Multivalent Ions, p. 97 Write the formula of tin(IV) oxide. must balance 4+ Sn 4+ 2— O 2—

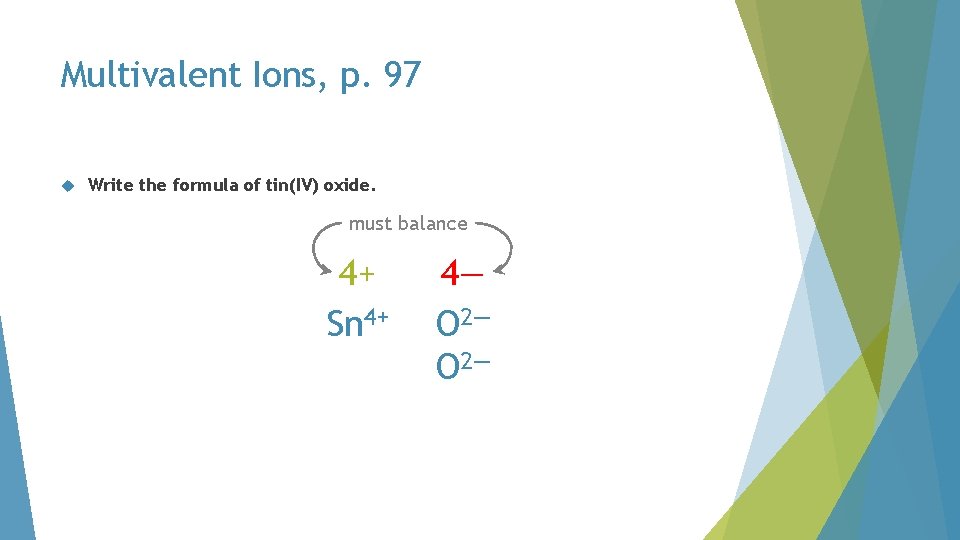
Multivalent Ions, p. 97 Write the formula of tin(IV) oxide. must balance 4+ Sn 4+ 4— O 2—
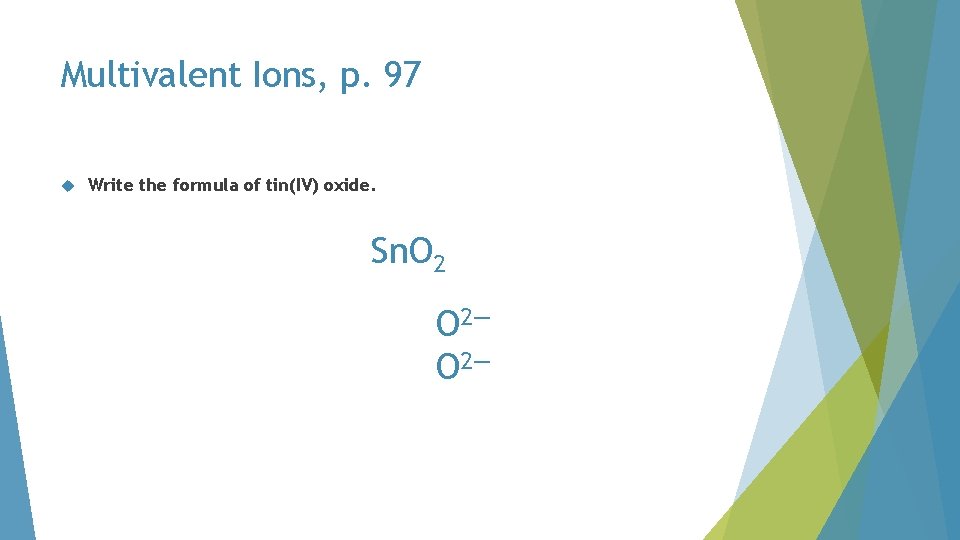
Multivalent Ions, p. 97 Write the formula of tin(IV) oxide. Sn. O 2—

single-valent metal multivalent metal monoatomic Zn. Cl 2 zinc chloride Cu 2 O copper(I) oxide

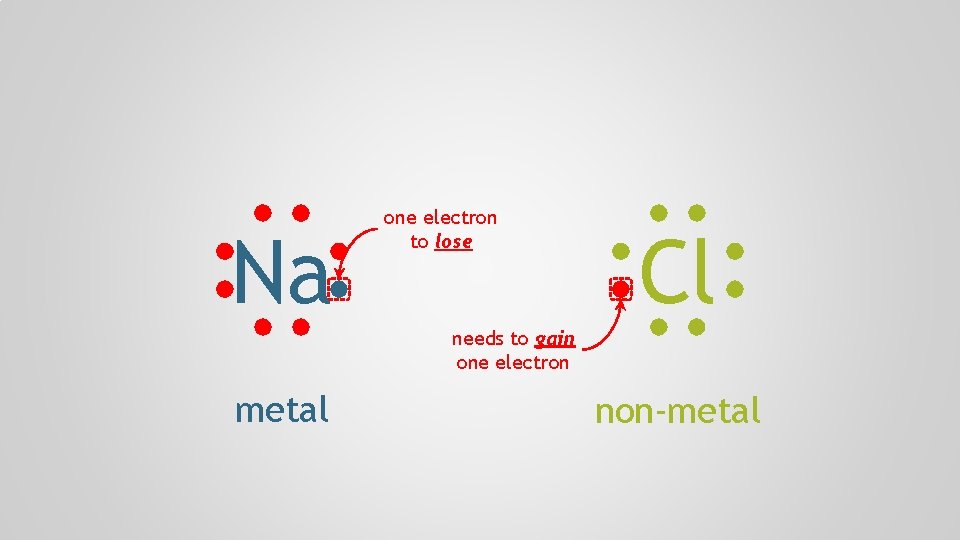
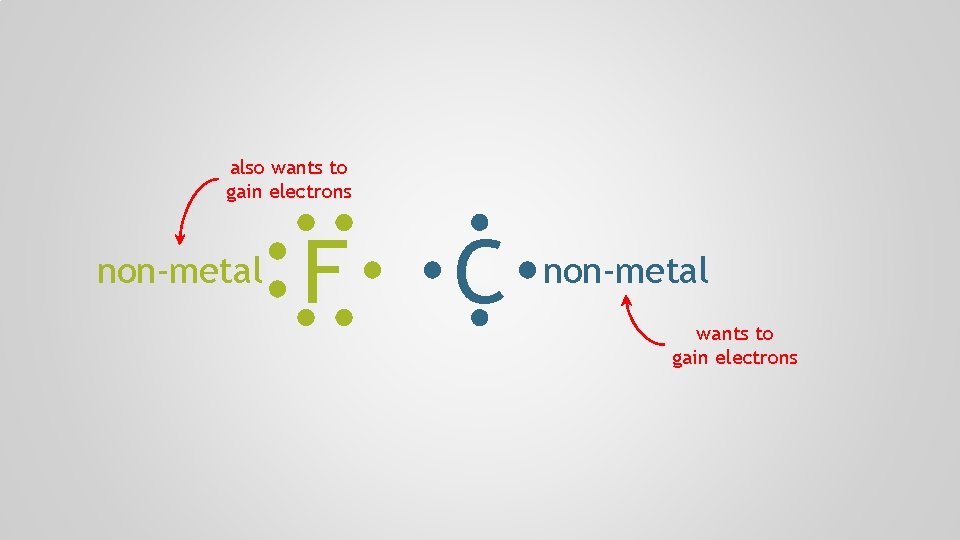
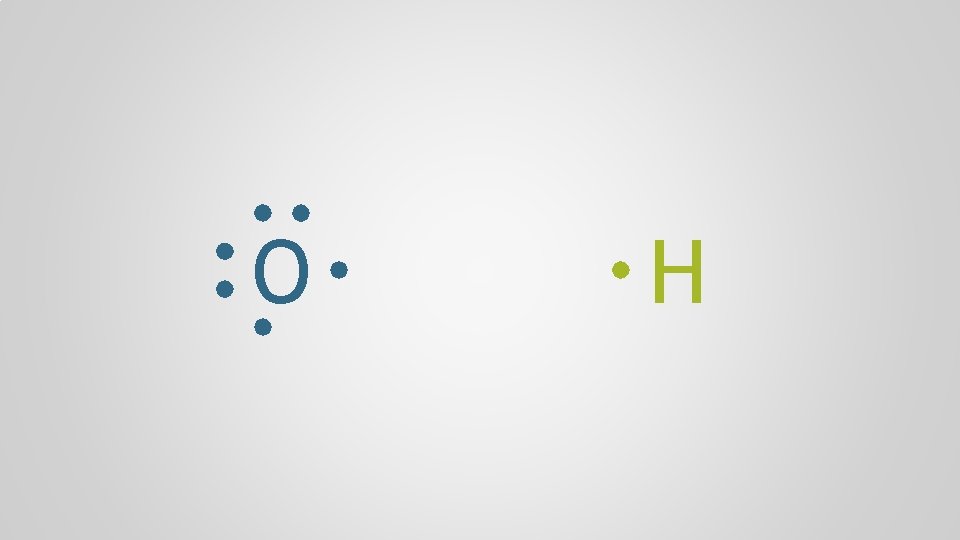
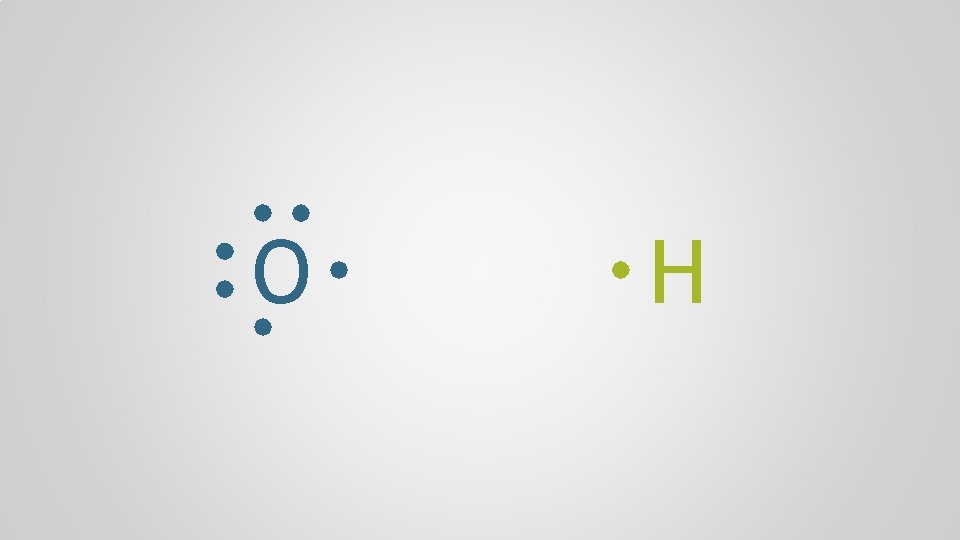
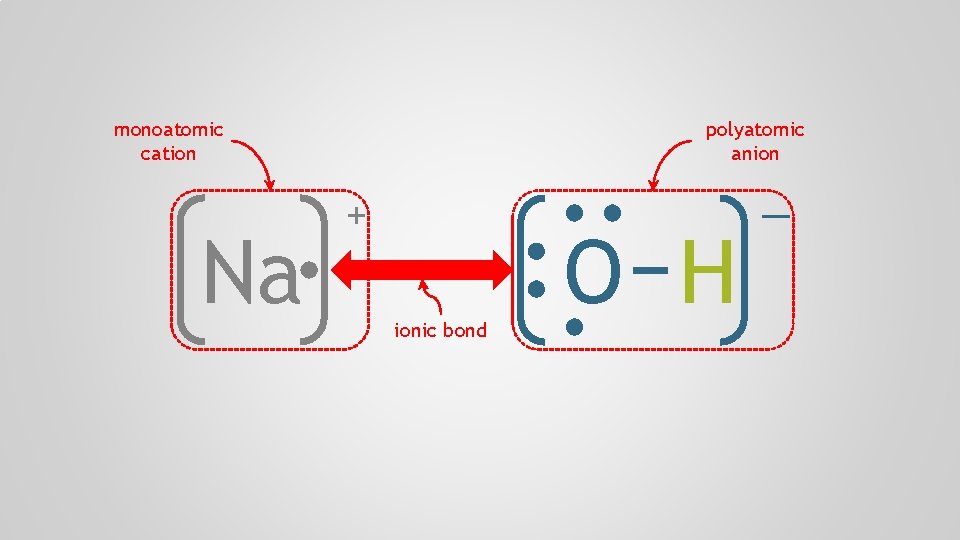
Polyatomic Ions, p. 98 Atoms form chemical bonds to achieve a full valence shell, by either filling the current one or by losing one that’s only partially occupied

Na one electron to lose Cl needs to gain one electron metal non-metal

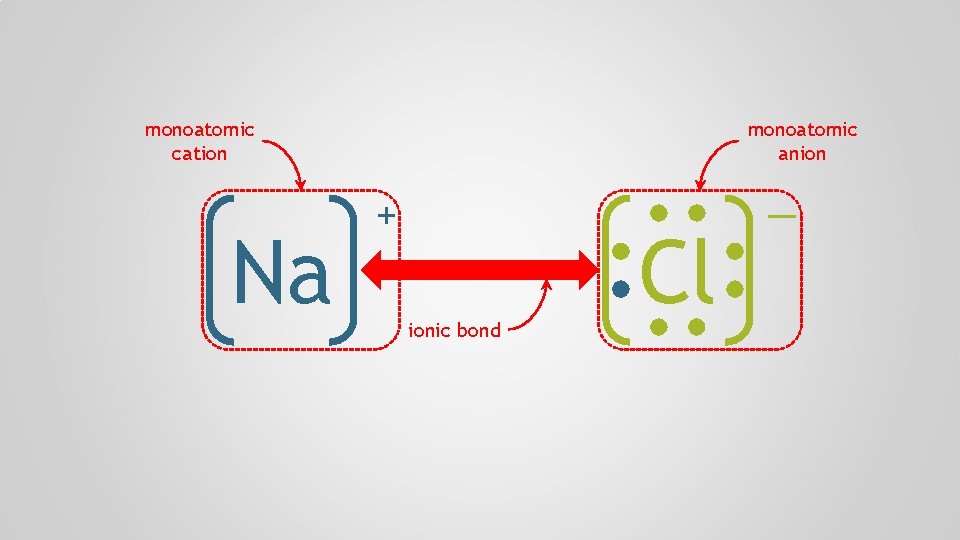
Na Cl

monoatomic cation Na monoatomic anion + ionic bond Cl —

also wants to gain electrons non-metal F C non-metal wants to gain electrons

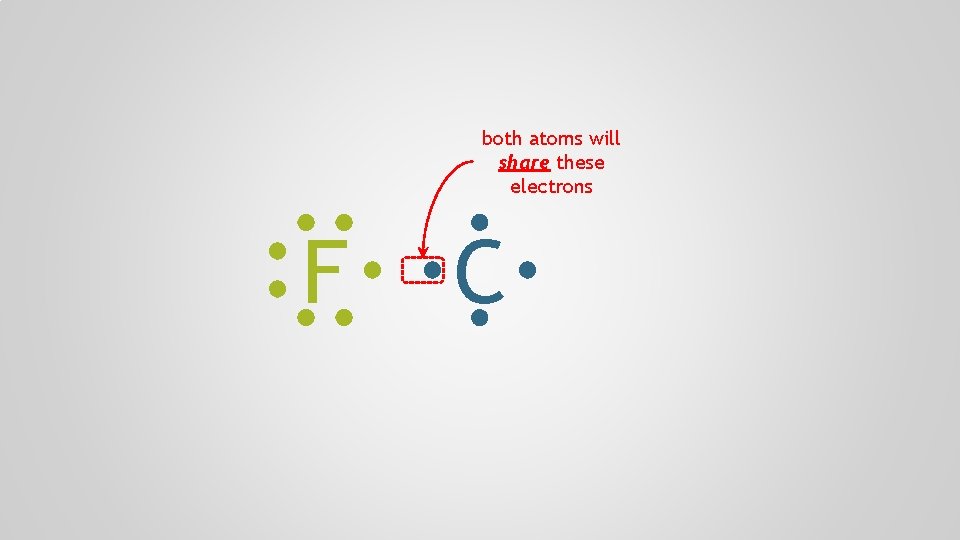
both atoms will share these electrons F C

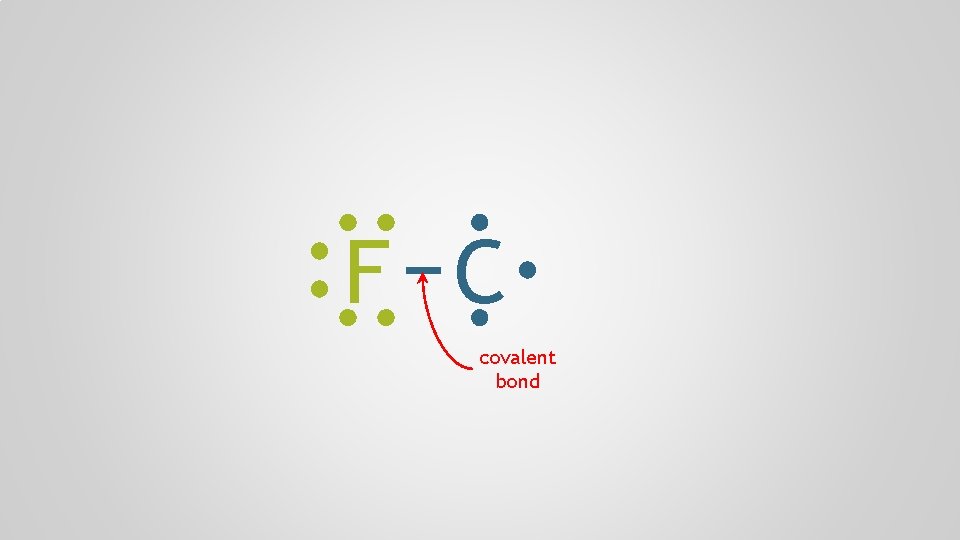
F C covalent bond

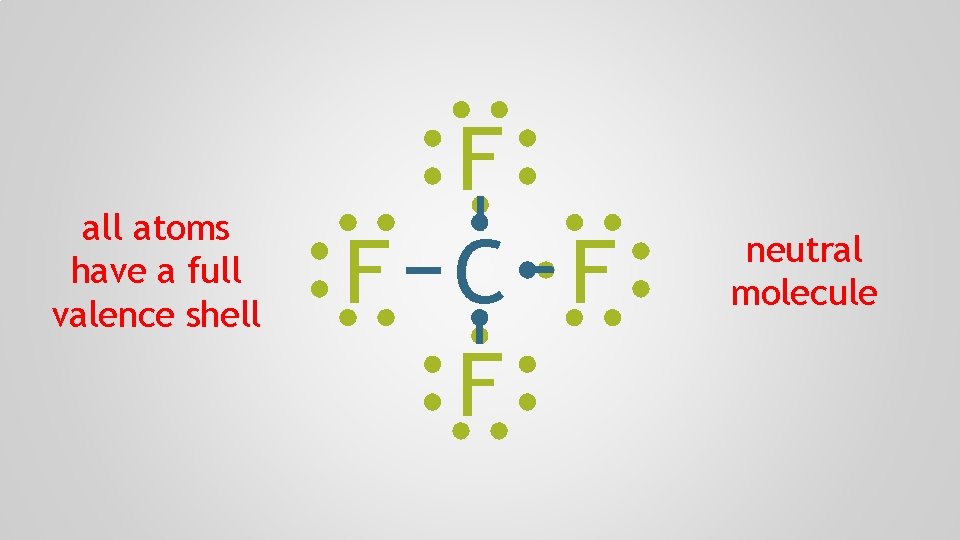
all atoms have a full valence shell F F C F F neutral molecule

O H

O H

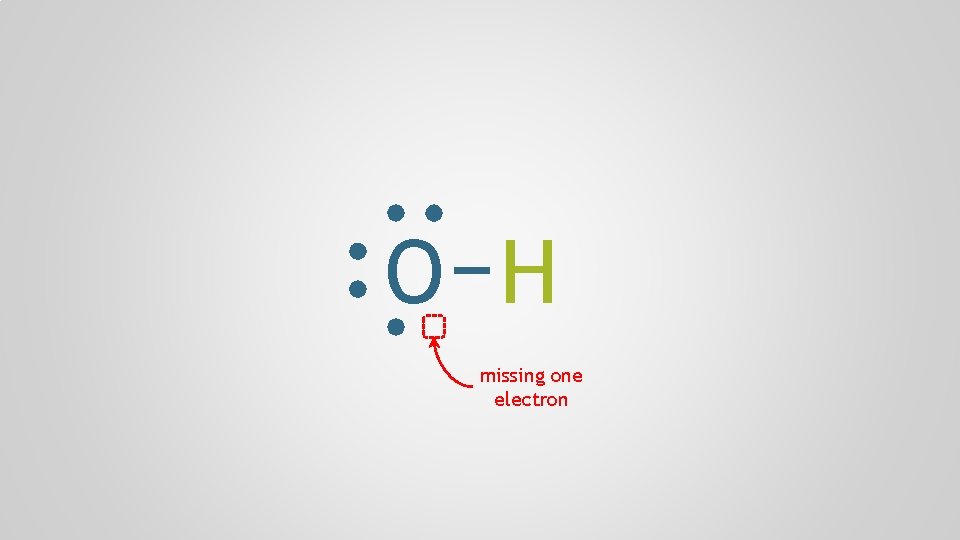
O H missing one electron

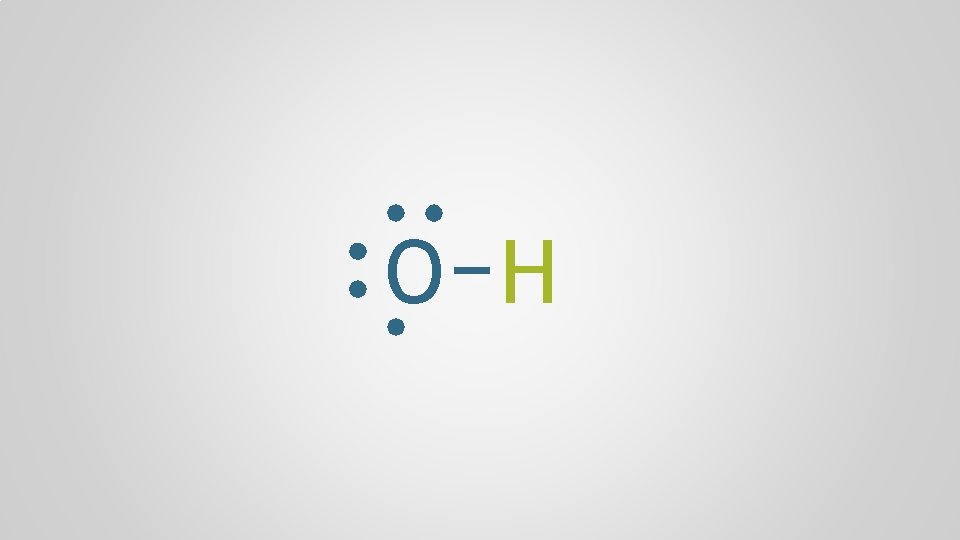
O H

monoatomic cation Na polyatomic anion + ionic bond O H —

Polyatomic Ions, p. 98 Polyatomic ion A group of atoms who bond together through electron sharing and covalent bonds, who collectively need to gain or lose one or more electrons, forming a molecular ion multiple + atom polyatomic

Polyatomic Ions, p. 98 Polyatomic ions often remain intact during a chemical reaction, appearing in a compound as a reactant and in a different compound as a product

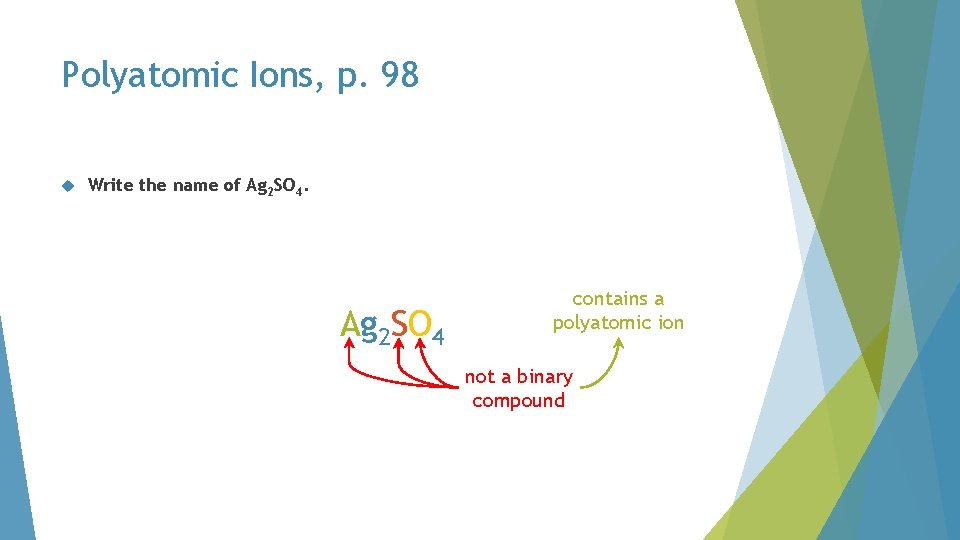
Polyatomic Ions, p. 98 The formula of an ionic compound containing a polyatomic ion has three or more capital letters, directing you to the table of polyatomic ions in the data booklet

Polyatomic Ions, p. 98 Write the name of Ag 2 SO 4. A Ag 2 SO 4 contains a polyatomic ion not a binary compound

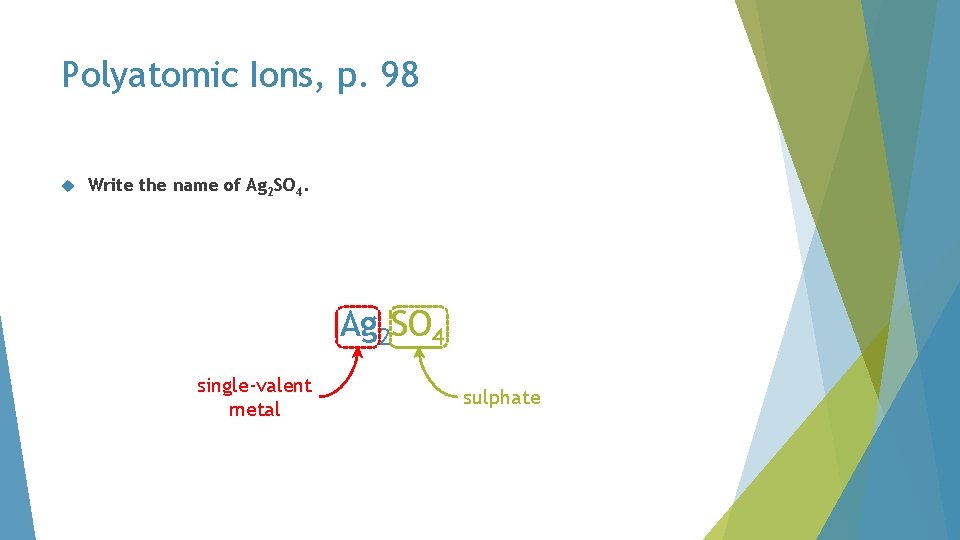
Polyatomic Ions, p. 98 Write the name of Ag 2 SO 4. Ag 2 SO SO 4 single-valent metal sulphate

Polyatomic Ions, p. 98 Write the name of Ag 2 SO 4

Polyatomic Ions, p. 98 Write the name of Ag 2 SO 4. Ag 2 sulphate

Polyatomic Ions, p. 98 Write the name of Ag 2 SO 4. silver sulphate

single-valent metal multivalent metal monoatomic Zn. Cl 2 zinc chloride Cu 2 O copper(I) oxide single-valent polyatomic Ag 2 SO 4 silver sulphate

Polyatomic Ions, p. 98 Polyatomic cations usually end with —onium, while polyatomic anions usually end with —ite or —ate

Polyatomic Ions, p. 98 When a compound’s formula contains multiples of a polyatomic ion, first surround the polyatomic’s formula to define it, then add a subscript number to multiply it

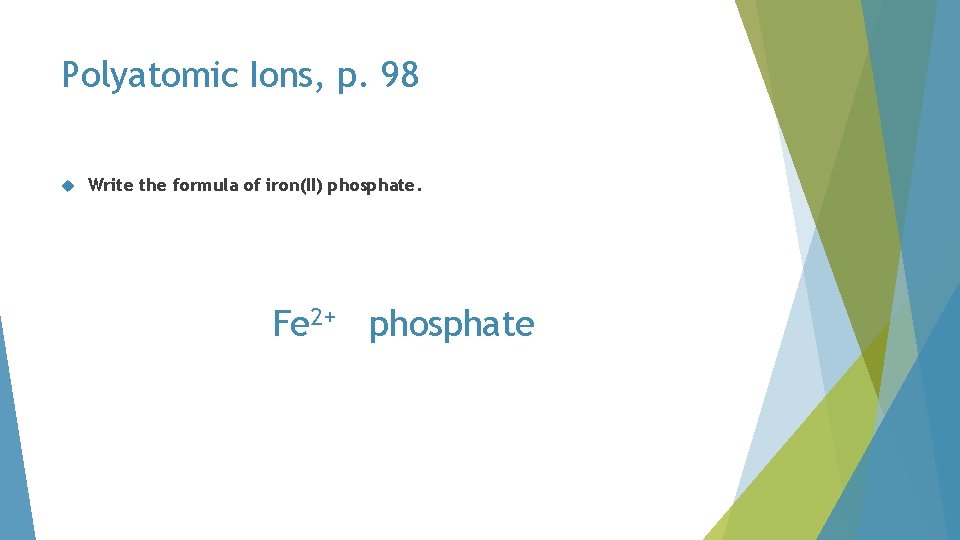
Polyatomic Ions, p. 98 Write the formula of iron(II) phosphate

Polyatomic Ions, p. 98 Write the formula of iron(II) phosphate. Fe 2+ phosphate

Polyatomic Ions, p. 98 Write the formula of iron(II) phosphate. must balance 2+ Fe 2+ 3— PO 43—
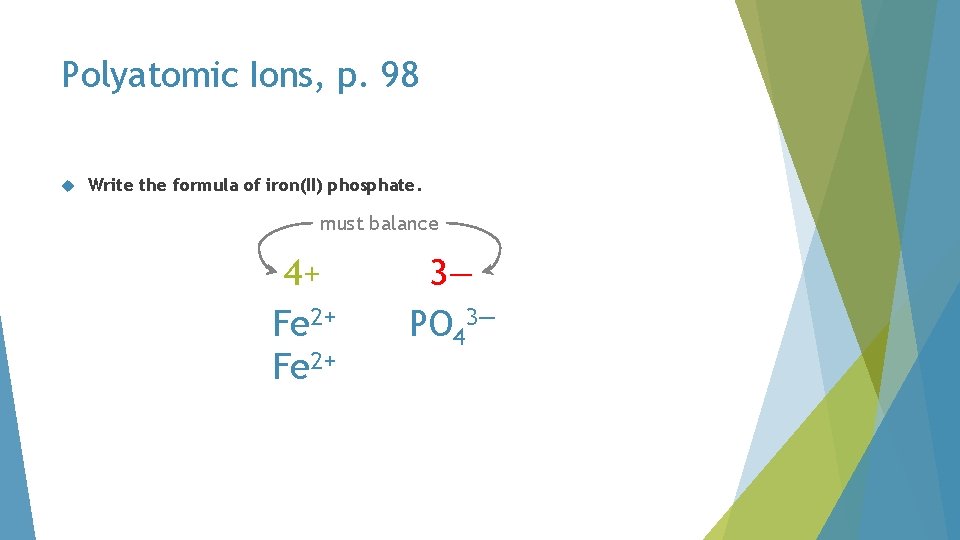
Polyatomic Ions, p. 98 Write the formula of iron(II) phosphate. must balance 4+ Fe 2+ 3— PO 43—

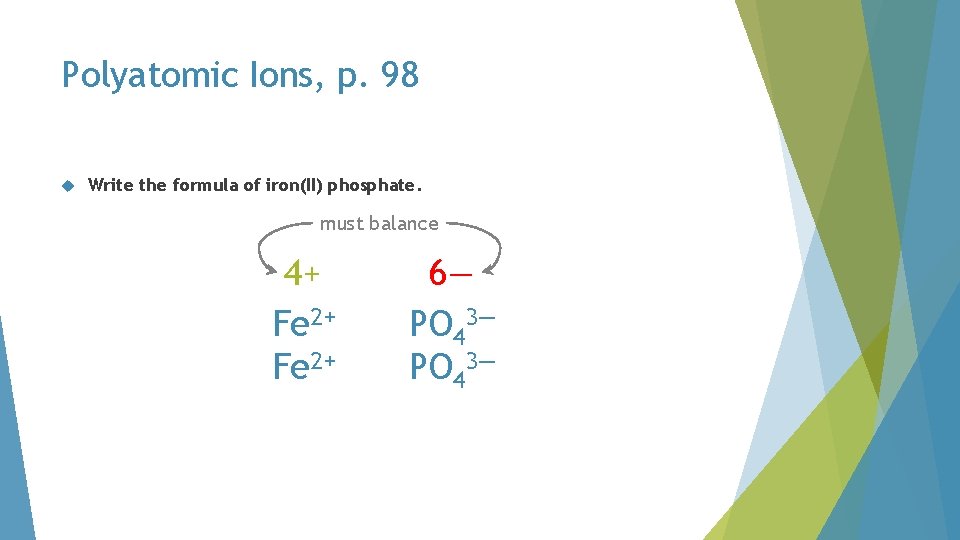
Polyatomic Ions, p. 98 Write the formula of iron(II) phosphate. must balance 4+ Fe 2+ 6— PO 43—

Polyatomic Ions, p. 98 Write the formula of iron(II) phosphate. must balance 6+ Fe 2+ 6— PO 43—
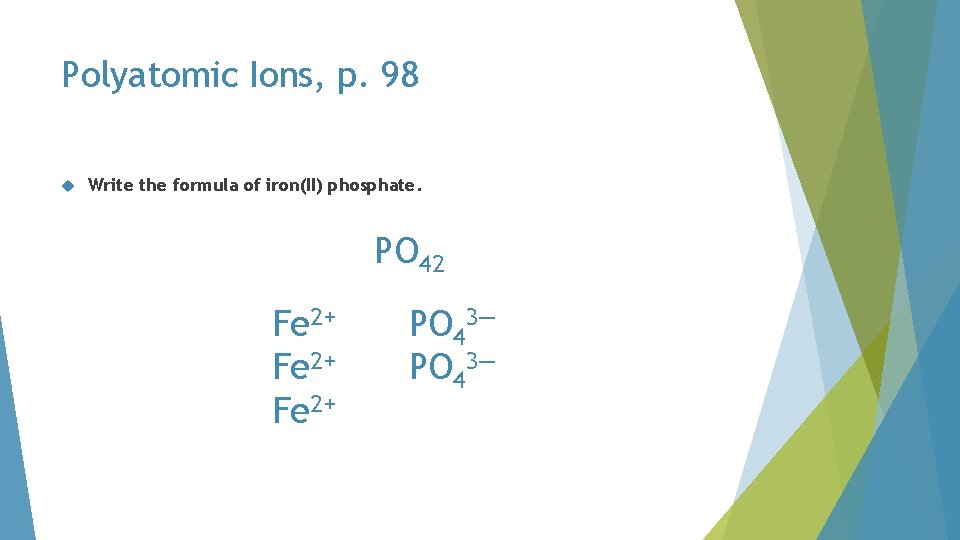
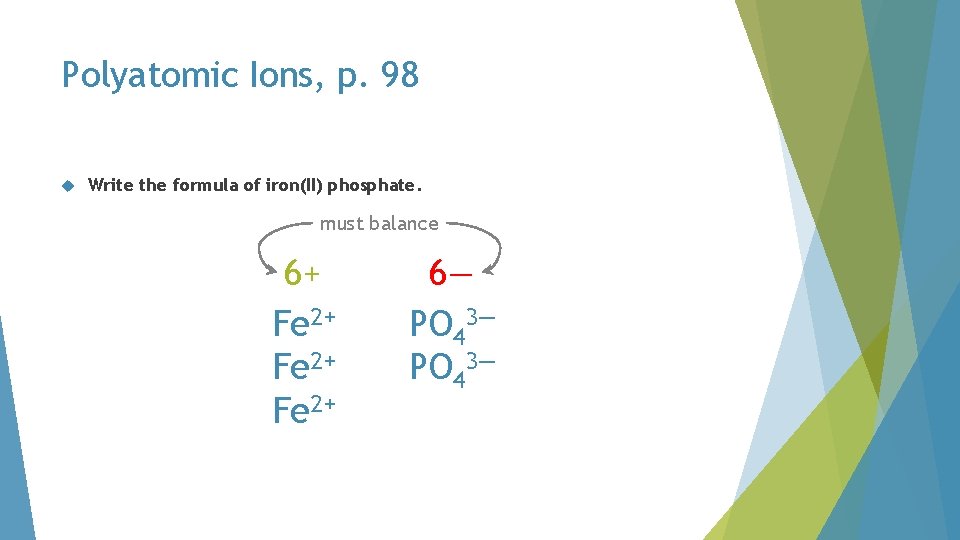
Polyatomic Ions, p. 98 Write the formula of iron(II) phosphate. PO 42 Fe 2+ PO 43—
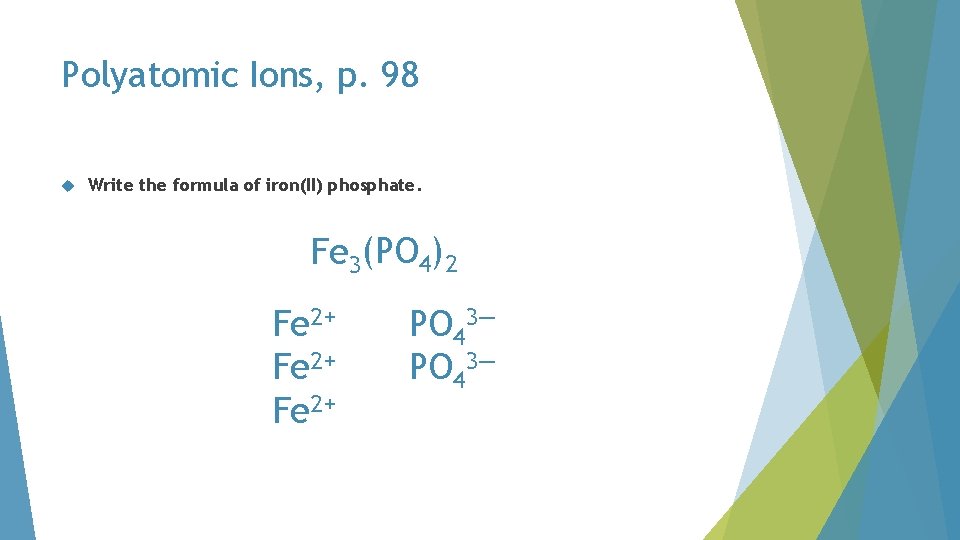
Polyatomic Ions, p. 98 Write the formula of iron(II) phosphate. Fe 3(PO 4)2 Fe 2+ PO 43—

single-valent metal multivalent metal monoatomic Zn. Cl 2 zinc chloride Cu 2 O copper(I) oxide single-valent multivalent metal polyatomic Ag 2 SO 4 silver sulphate Fe 3(PO 4)2 iron(II) phosphate

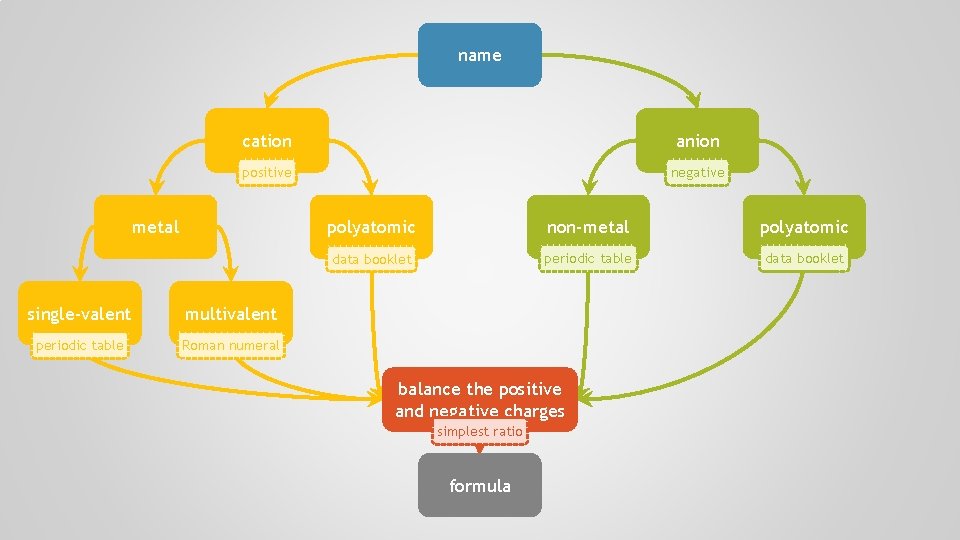
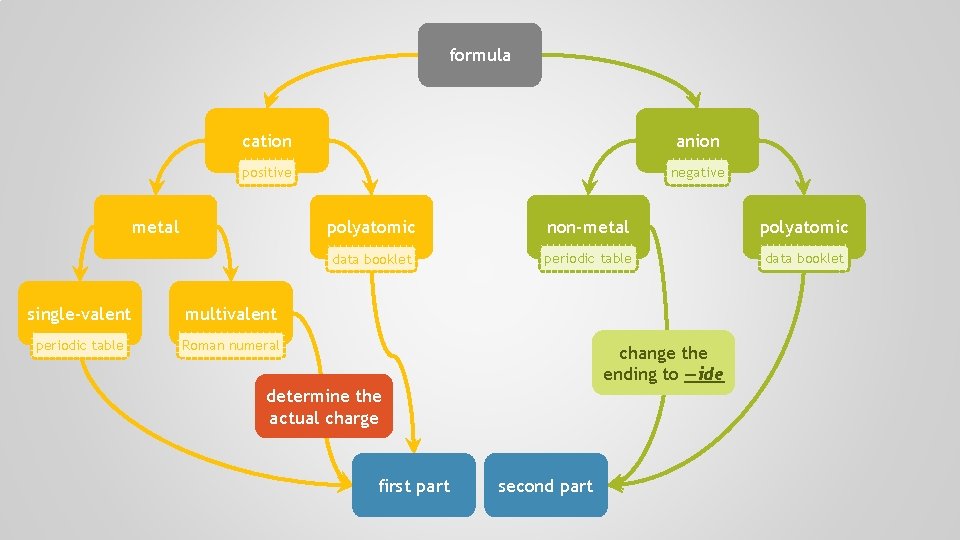
name cation anion positive negative metal single-valent multivalent periodic table Roman numeral polyatomic non-metal polyatomic data booklet periodic table data booklet balance the positive and negative charges simplest ratio formula

formula name

formula cation anion positive negative metal single-valent multivalent periodic table Roman numeral polyatomic non-metal polyatomic data booklet periodic table data booklet change the ending to —ide determine the actual charge first part second part

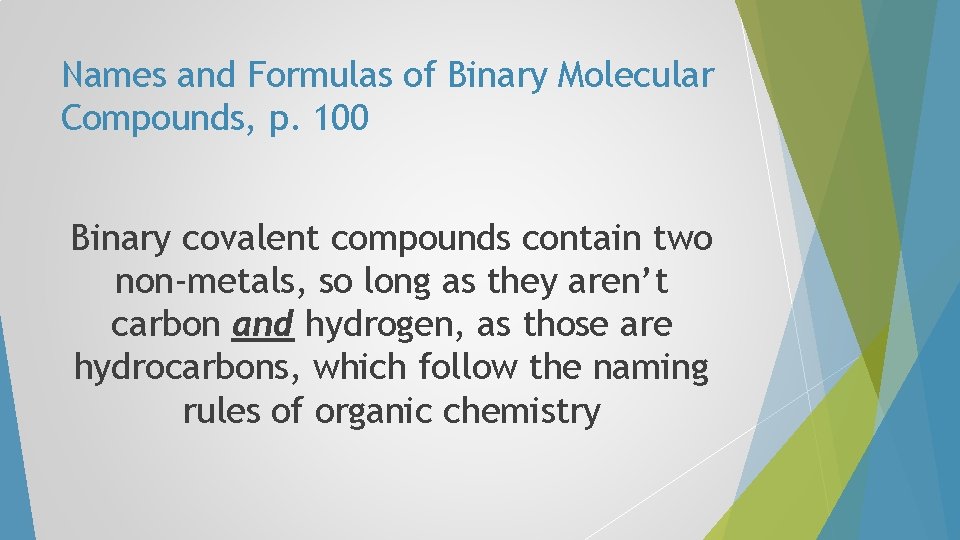
Names and Formulas of Binary Molecular Compounds, p. 100 Binary covalent compounds contain two non-metals, so long as they aren’t carbon and hydrogen, as those are hydrocarbons, which follow the naming rules of organic chemistry

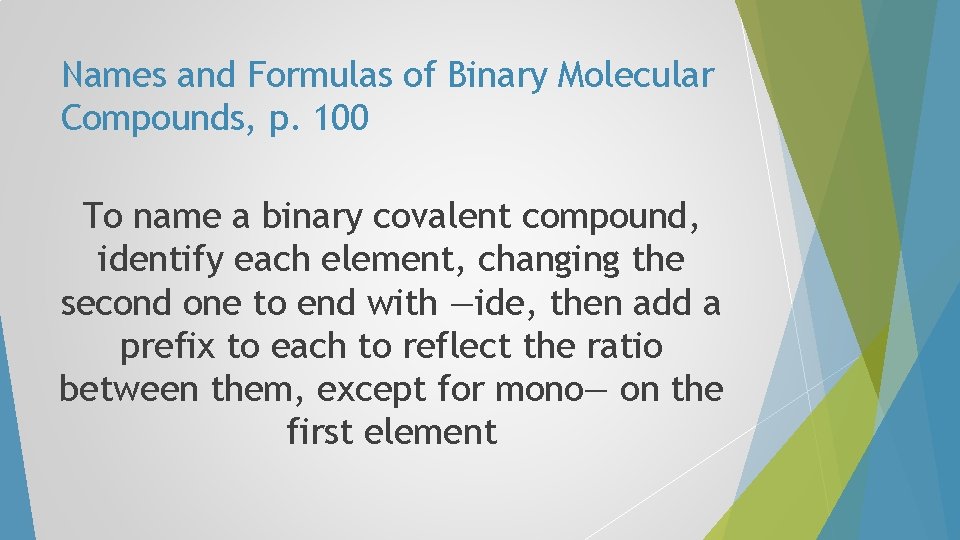
Names and Formulas of Binary Molecular Compounds, p. 100 To name a binary covalent compound, identify each element, changing the second one to end with —ide, then add a prefix to each to reflect the ratio between them, except for mono— on the first element

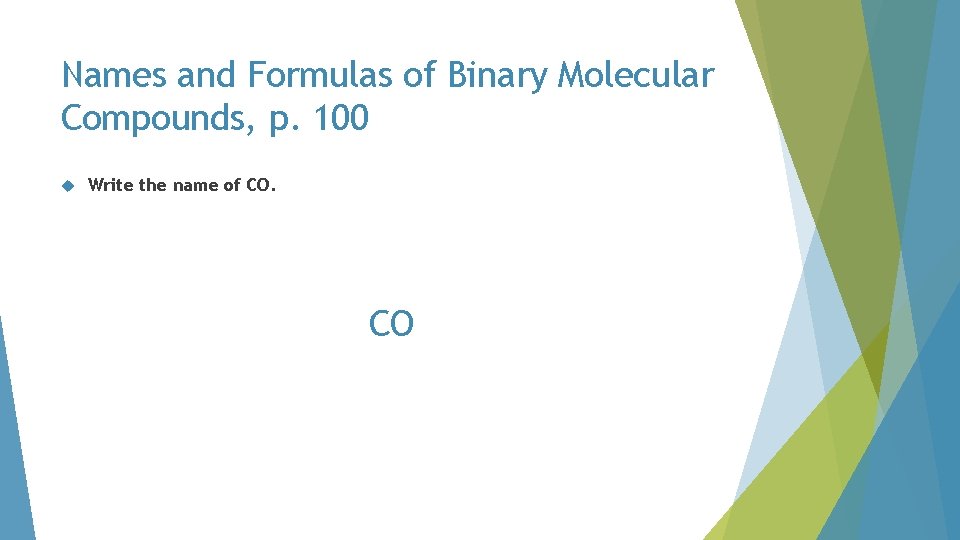
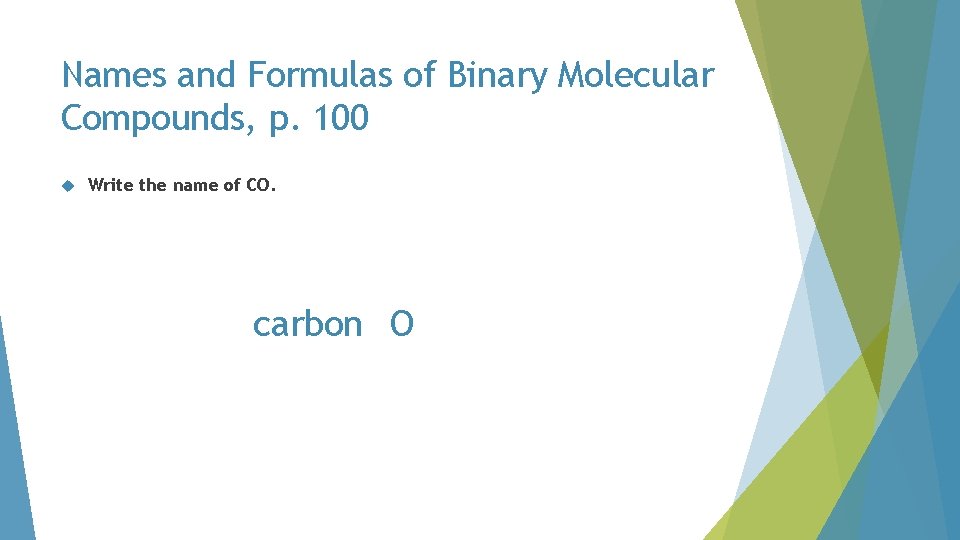
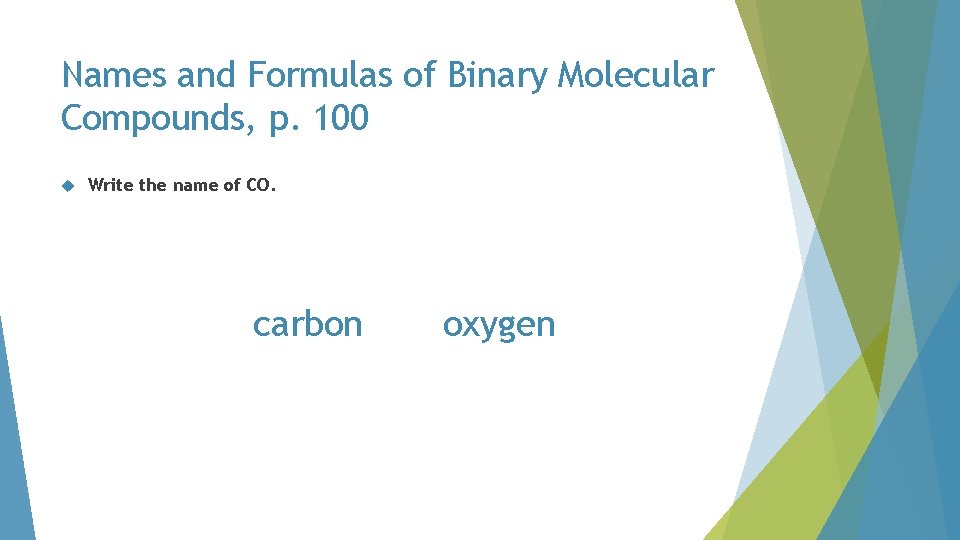
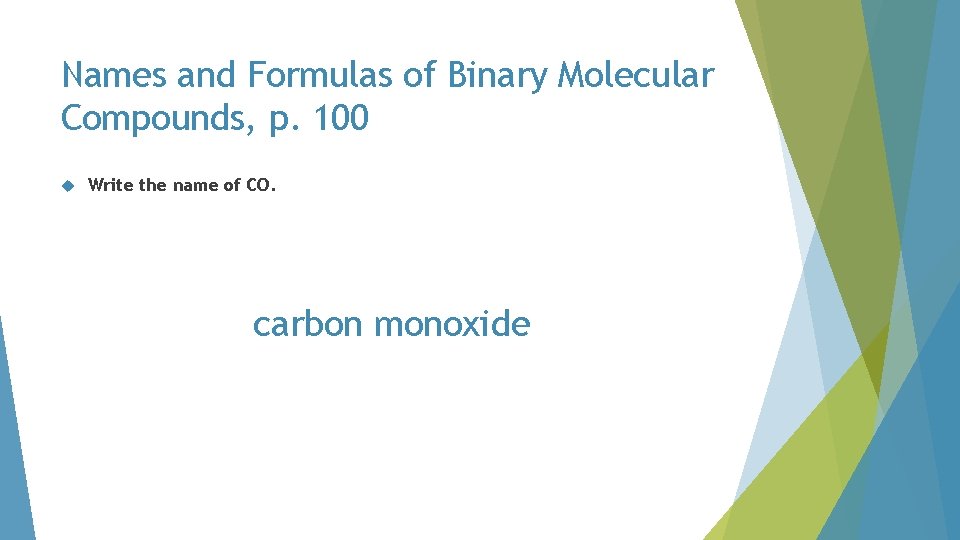
Names and Formulas of Binary Molecular Compounds, p. 100 Write the name of CO. CO

Names and Formulas of Binary Molecular Compounds, p. 100 Write the name of CO. carbon O

Names and Formulas of Binary Molecular Compounds, p. 100 Write the name of CO. carbon oxygen

Names and Formulas of Binary Molecular Compounds, p. 100 Write the name of CO. carbon monoxide

Names and Formulas of Binary Molecular Compounds, p. 100 To translate a binary covalent compound’s name into a formula, identify each element’s symbol, then convert any prefixes to subscripts, remembering that no prefix and mono— don’t generate a number

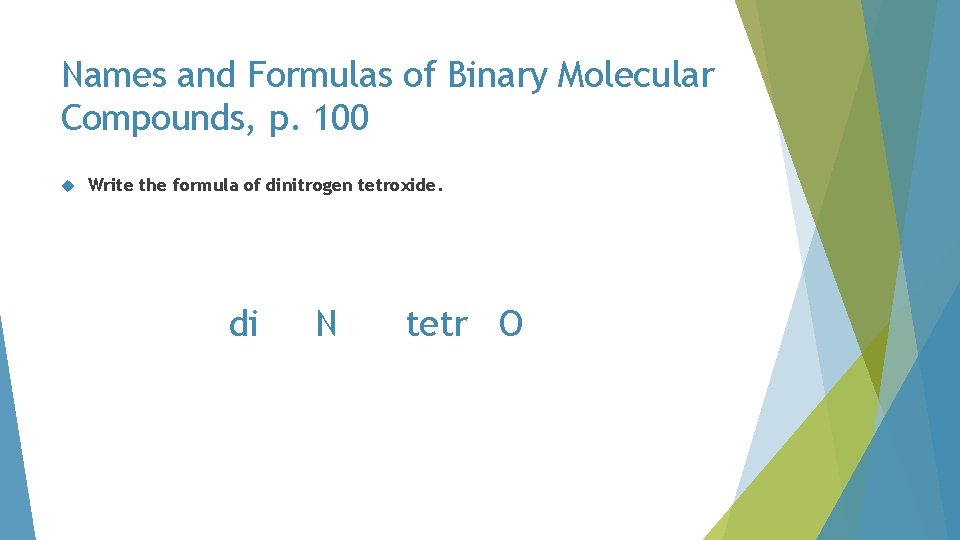
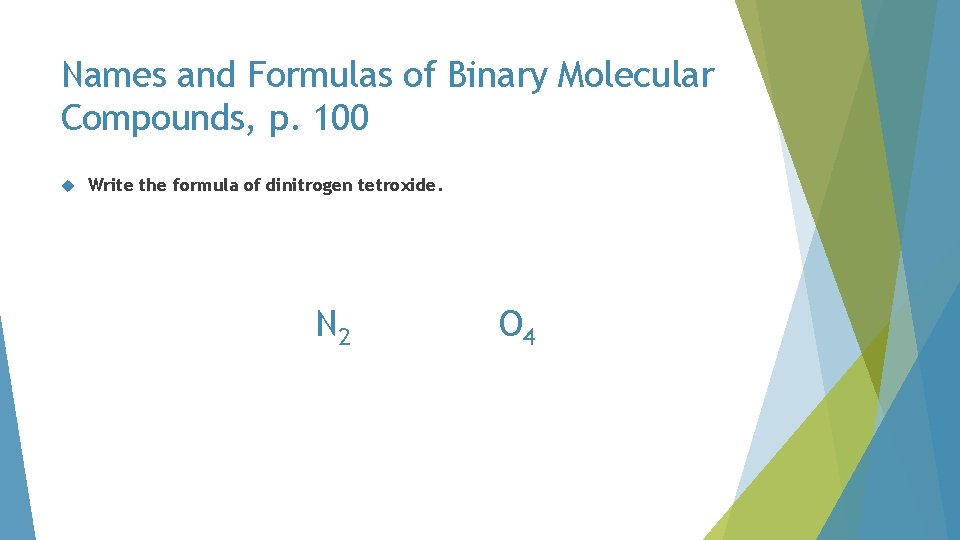
Names and Formulas of Binary Molecular Compounds, p. 100 Write the formula of dinitrogen tetroxide

Names and Formulas of Binary Molecular Compounds, p. 100 Write the formula of dinitrogen tetroxide. di N tetr O

Names and Formulas of Binary Molecular Compounds, p. 100 Write the formula of dinitrogen tetroxide. N 2 O 4