2 4 Chronoamperometry measurement of currents as a







- Slides: 19



2. 4. Chronoamperometry ümeasurement of currents as a function of time üa kind of ‘controlled-potential voltammetry’ or ‘controlledpotential micro electrolysis (unstirred solution)’ üUse of the same equipment of cyclic voltammetry üWhen cyclic voltammetry does not succeed in identifying the electrode mechanisms

Single potential step Chronoamperometry Ox + ne - Red 1. applying an appropriate potential (under stationary conditions similar to those of cyclic voltammetry), which allows the electron transfer to run instantaneously to completion (COx (0, t) → 0) 2. decay of the generated current is monitored

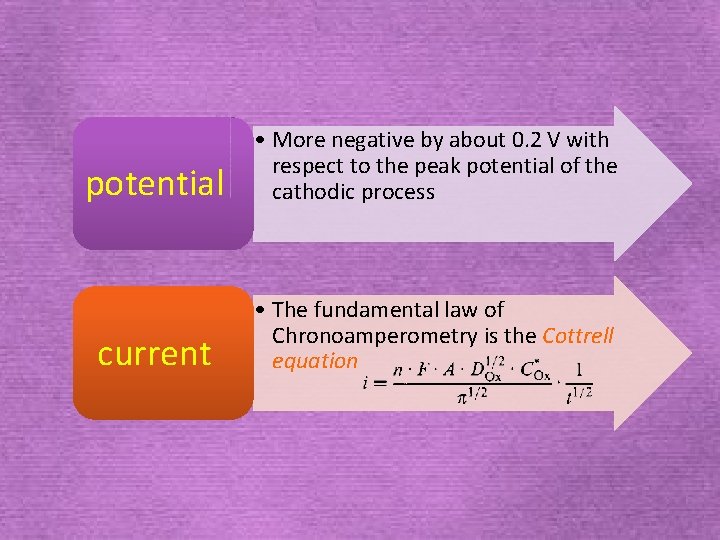
potential • More negative by about 0. 2 V with respect to the peak potential of the cathodic process current • The fundamental law of Chronoamperometry is the Cottrell equation

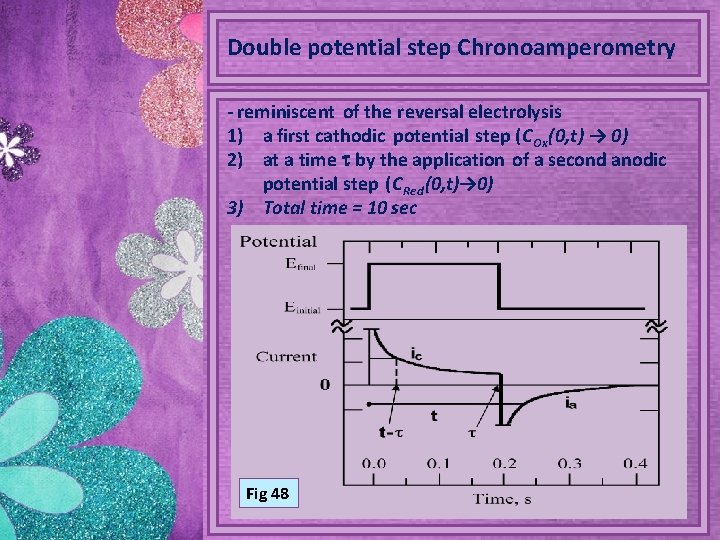
Double potential step Chronoamperometry - reminiscent of the reversal electrolysis 1) a first cathodic potential step (C Ox(0, t) → 0) 2) at a time t by the application of a second anodic potential step (C Red (0, t)→ 0) 3) Total time = 10 sec Fig 48

t<t diagram represents the chronoamperometric single potential step response (Cottrell equation) t>t reoxidation of Red, diagram represents the double potential step response Fig 49 possible presence of coupled chemical reactions

2. 4. 1. Coupled Chemical Reactions

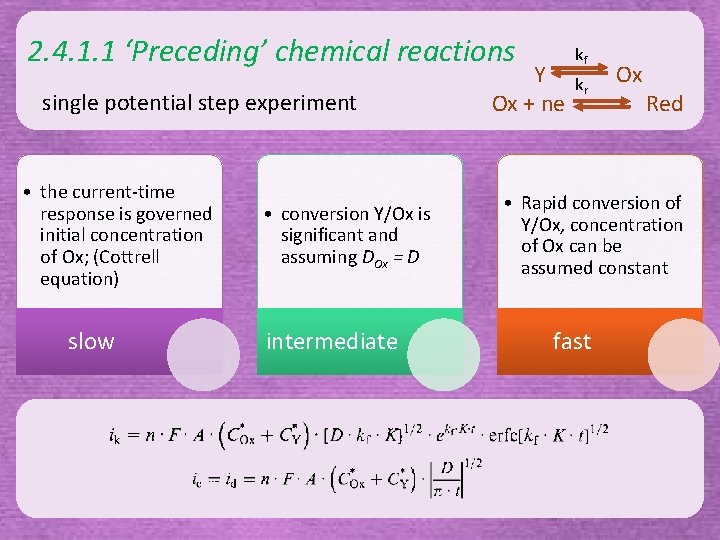
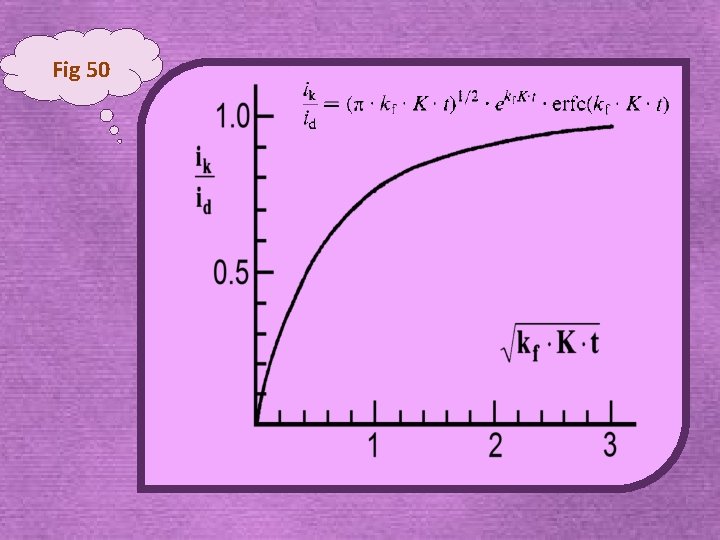
2. 4. 1. 1 ‘Preceding’ chemical reactions single potential step experiment • the current-time response is governed initial concentration of Ox; (Cottrell equation) slow • conversion Y/Ox is significant and assuming DOx = D intermediate Y Ox + ne kf kr Ox Red • Rapid conversion of Y/Ox, concentration of Ox can be assumed constant fast

Fig 50

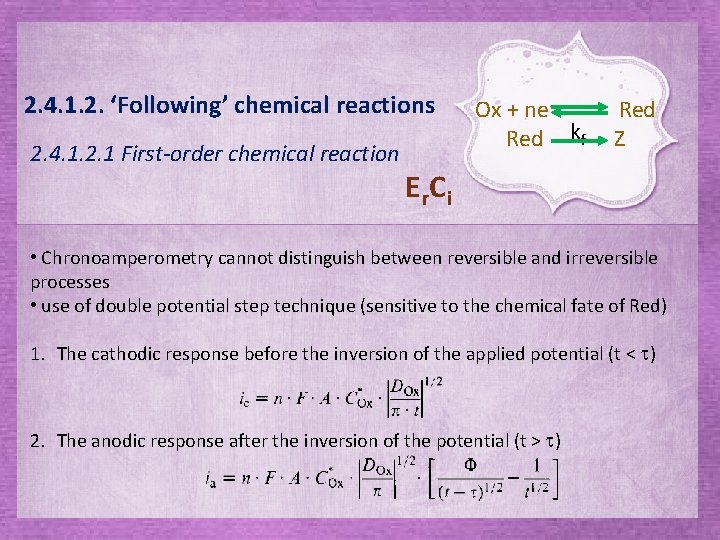
2. 4. 1. 2. ‘Following’ chemical reactions 2. 4. 1. 2. 1 First-order chemical reaction Ox + ne. Red kf Red Z E r. C i • Chronoamperometry cannot distinguish between reversible and irreversible processes • use of double potential step technique (sensitive to the chemical fate of Red) 1. The cathodic response before the inversion of the applied potential (t < t) 2. The anodic response after the inversion of the potential (t > t)

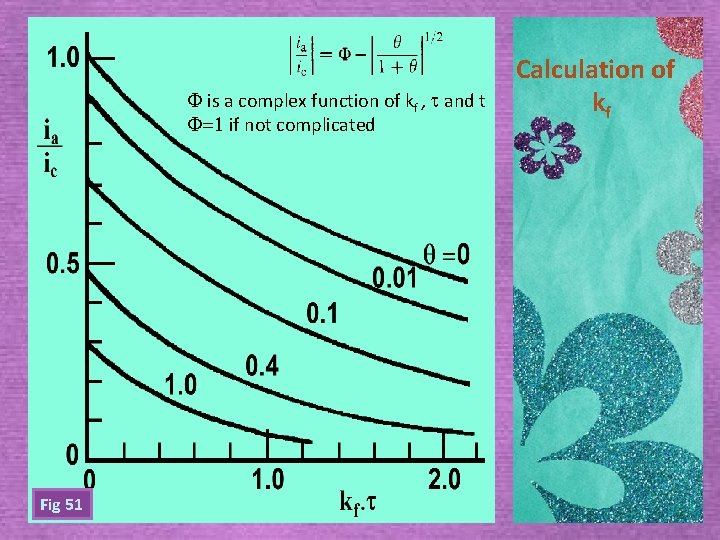
F is a complex function of kf , t and t F=1 if not complicated Fig 51 Calculation of kf

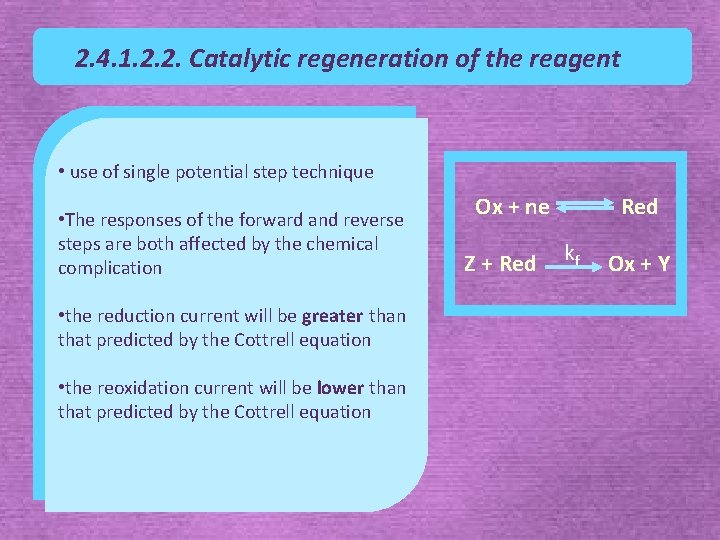
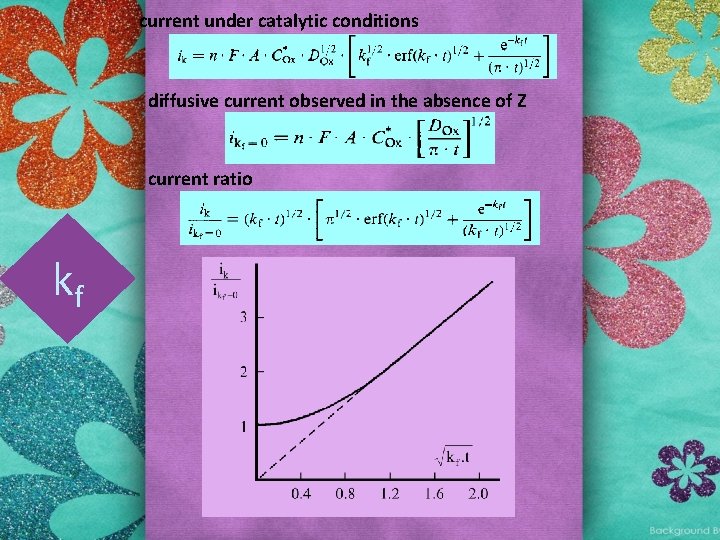
2. 4. 1. 2. 2. Catalytic regeneration of the reagent • use of single potential step technique • The responses of the forward and reverse steps are both affected by the chemical complication • the reduction current will be greater than that predicted by the Cottrell equation • the reoxidation current will be lower than that predicted by the Cottrell equation Ox + ne Z + Red kf Ox + Y

current under catalytic conditions diffusive current observed in the absence of Z current ratio kf

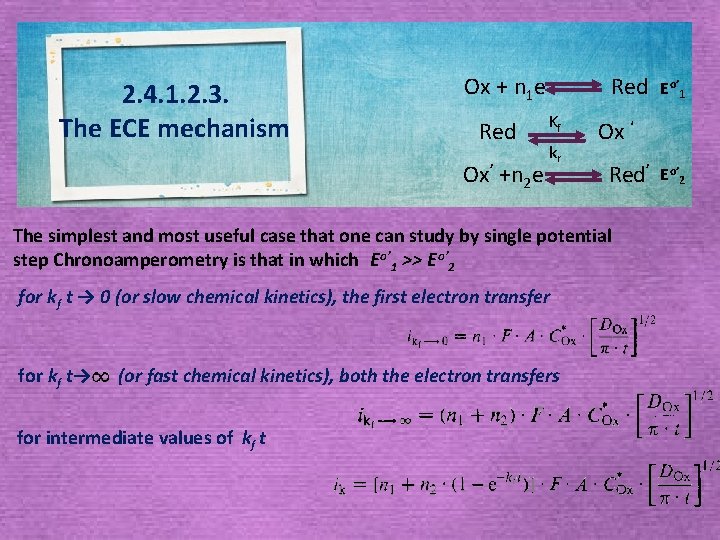
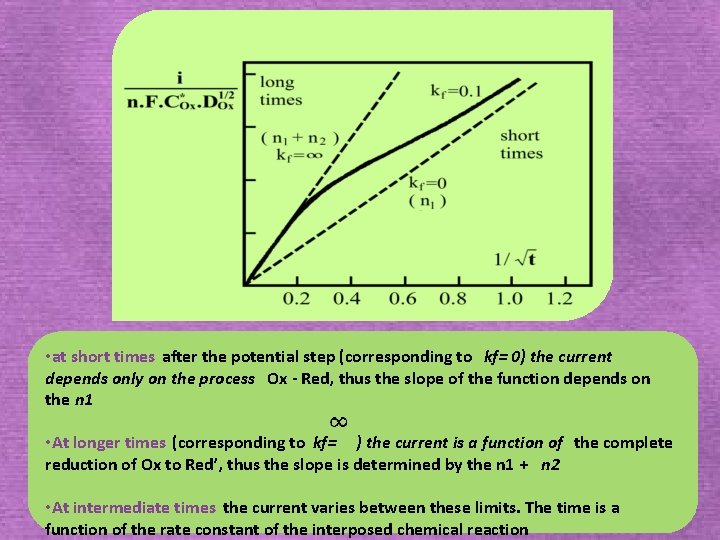
2. 4. 1. 2. 3. The ECE mechanism Ox + n 1 e Red Ox’ +n 2 e Red Kf kr Ox ‘ Red’ The simplest and most useful case that one can study by single potential step Chronoamperometry is that in which Eo’ 1 >> E o’ 2 for k f t → 0 (or slow chemical kinetics), the first electron transfer for kf t→ (or fast chemical kinetics), both the electron transfers for intermediate values of kf t Eo’ 1 Eo’ 2

• at short times after the potential step (corresponding to kf= 0) the current depends only on the process Ox - Red, thus the slope of the function depends on the n 1 • At longer times (corresponding to kf= ) the current is a function of the complete reduction of Ox to Red’, thus the slope is determined by the n 1 + n 2 • At intermediate times the current varies between these limits. The time is a function of the rate constant of the interposed chemical reaction

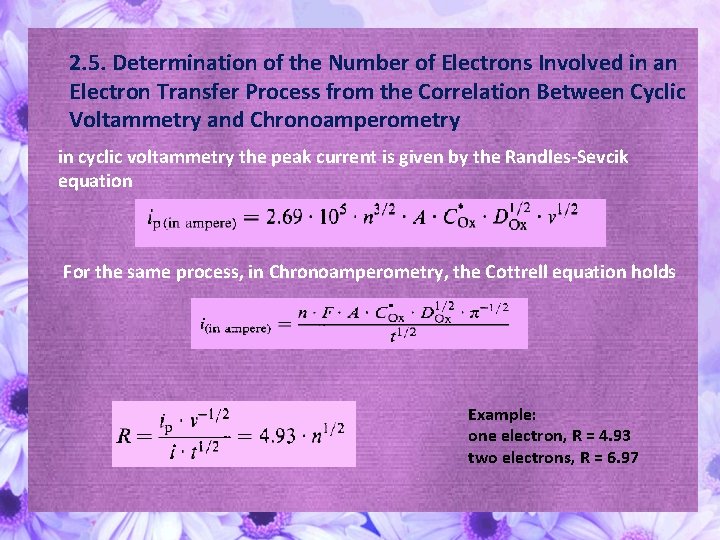
2. 5. Determination of the Number of Electrons Involved in an Electron Transfer Process from the Correlation Between Cyclic Voltammetry and Chronoamperometry in cyclic voltammetry the peak current is given by the Randles-Sevcik equation For the same process, in Chronoamperometry, the Cottrell equation holds Example: one electron, R = 4. 93 two electrons, R = 6. 97

That ′s all. .
