2 4 Chemical Reactions KEY CONCEPT Life depends

- Slides: 7

2. 4 Chemical Reactions KEY CONCEPT Life depends on chemical reactions.

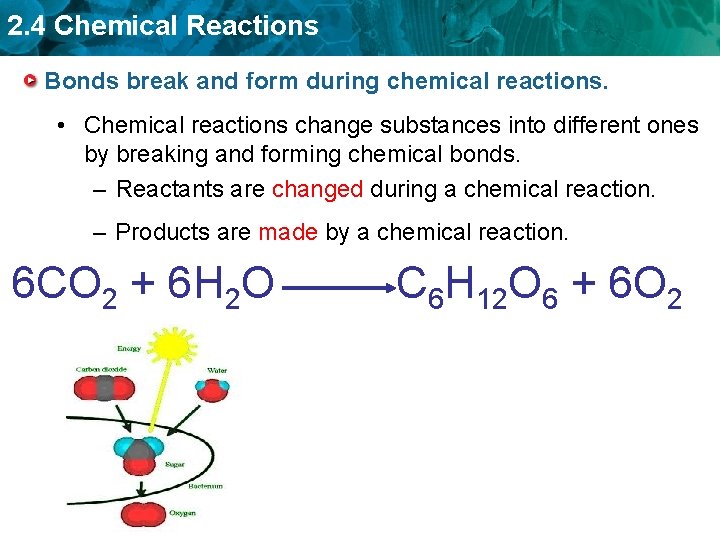
2. 4 Chemical Reactions Bonds break and form during chemical reactions. • Chemical reactions change substances into different ones by breaking and forming chemical bonds. – Reactants are changed during a chemical reaction. – Products are made by a chemical reaction. 6 CO 2 + 6 H 2 O C 6 H 12 O 6 + 6 O 2

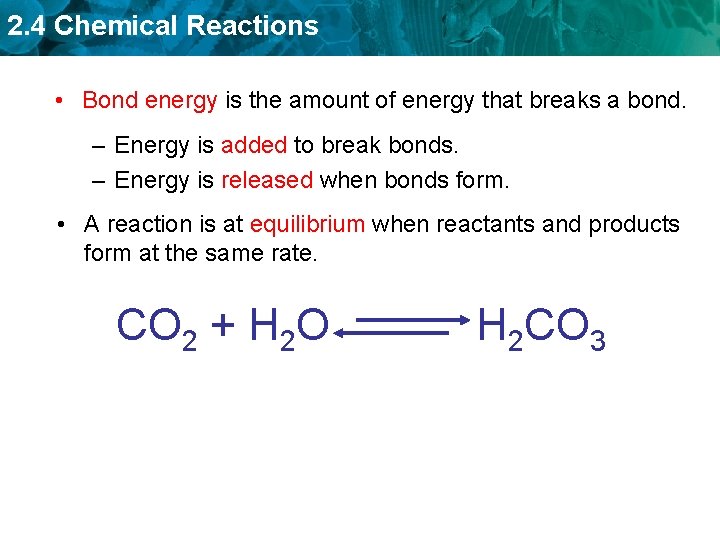
2. 4 Chemical Reactions • Bond energy is the amount of energy that breaks a bond. – Energy is added to break bonds. – Energy is released when bonds form. • A reaction is at equilibrium when reactants and products form at the same rate. CO 2 + H 2 O H 2 CO 3

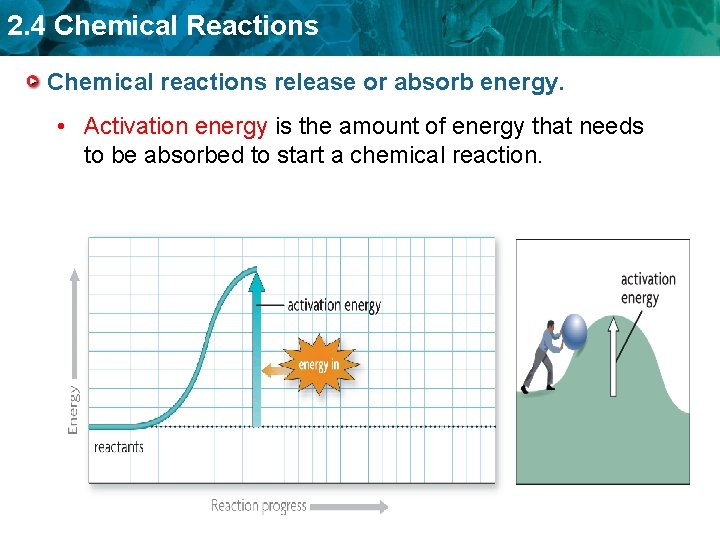
2. 4 Chemical Reactions Chemical reactions release or absorb energy. • Activation energy is the amount of energy that needs to be absorbed to start a chemical reaction.

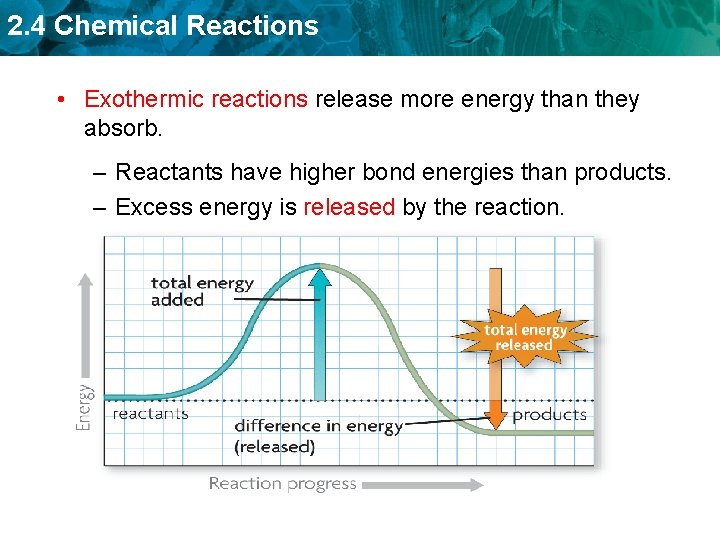
2. 4 Chemical Reactions • Exothermic reactions release more energy than they absorb. – Reactants have higher bond energies than products. – Excess energy is released by the reaction.

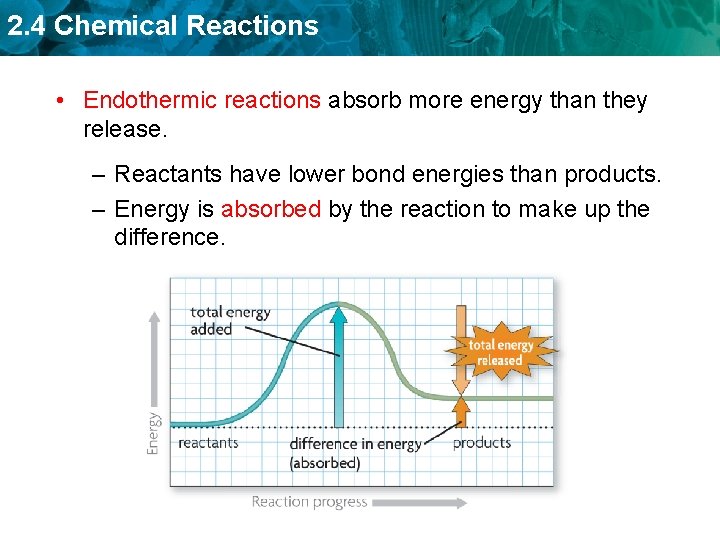
2. 4 Chemical Reactions • Endothermic reactions absorb more energy than they release. – Reactants have lower bond energies than products. – Energy is absorbed by the reaction to make up the difference.

2. 4 Chemical Reactions 2. 4 Review 1. Hydrogen peroxide (H 2 O 2) breaks down into water (H 2 O) and oxygen (O 2). Explain why this is a chemical reaction. What are the reactants and the products in this reaction? 2. How do endothermic and exothermic reactions differ? 3. The process below is exothermic. What must be true about the bond energies of the reactants and the products? Explain. 6 O 2 + C 6 H 12 O 6 6 CO 2 + 6 H 2 O 4. A chemical reaction can start when enough activation energy is added to the reactants. Do you think the activation energy for chemical reactions in living things is high or low? Explain.