2 3 Physical Properties of Minerals Each mineral













- Slides: 16

2. 3 – Physical Properties of Minerals Each mineral has specific properties that are the result of its chemical composition and crystalline structure. These properties provide useful clues for identifying minerals. Many of these properties can be identified by simply looking at a sample of the mineral or through simple tests.

Color color: what color the surface appears property is easily observed but is VERY unreliable because minerals can be affected by impurities or weathering

Streak streak: color of a mineral in powdered form more reliable than color for identification rub the mineral against an unglazed tile called a streak plate

Luster luster: way a mineral reflects light metallic: reflects light like a polished metal

nonmetallic: doesn’t reflect light like a metal glassy pearly earthy (dull)

Cleavage & Fracture cleavage: mineral breaks along planes of weakness to form smooth, flat surfaces

fracture: mineral breaks along irregular, rough surfaces (not flat!) conchoidal: breaks in a circular pattern (like chipped glass)

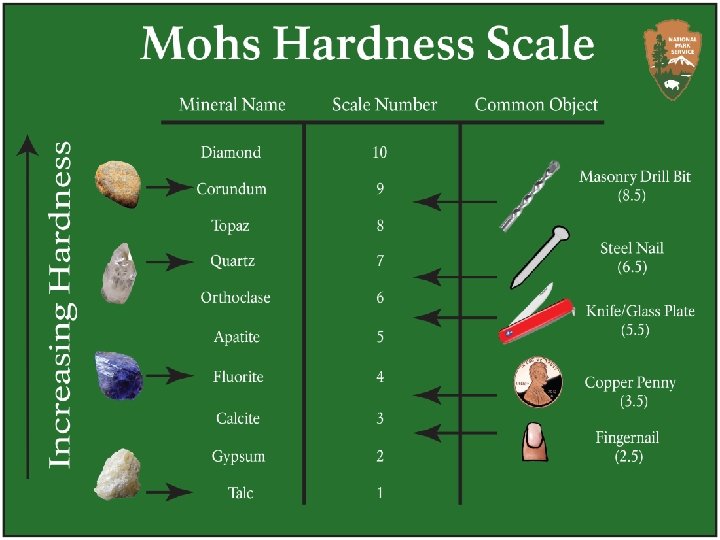
Hardness hardness: ability of a mineral to resist scratching Mohs hardness scale: scale against which the hardness of minerals is rated v 1 = softest v 10 = hardest strength of the bonds that make up a mineral’s structure determines the hardness of that mineral


to calculate hardness fingernail: 2. 5 penny: 3. 5 glass plate: 5. 5 vtry to scratch mineral with fingernail – if it can the hardness is “less than 2. 5”; if not, try with penny vif penny scratches mineral, hardness is “ 2. 5 – 3. 5”; if not, try the glass plate vtry to scratch the glass with the mineral – if it can’t then the hardness is “ 3. 5 – 5. 5”; if it can the hardness is “greater than 5. 5”

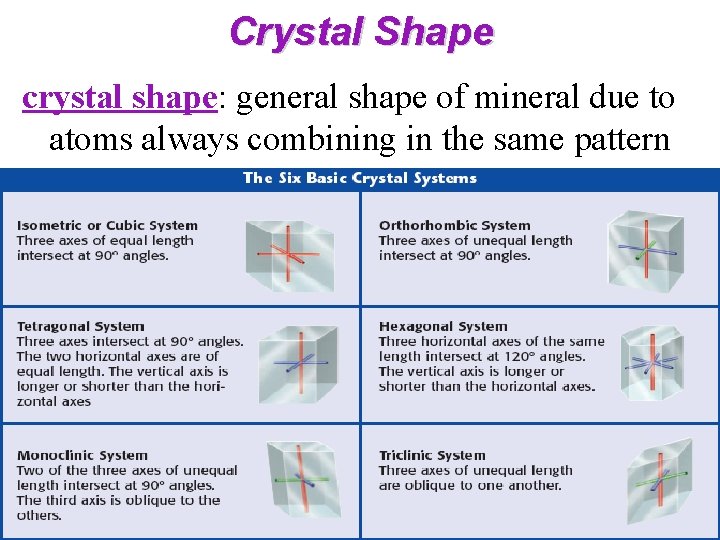
Crystal Shape crystal shape: general shape of mineral due to atoms always combining in the same pattern

Density (Specific Gravity) density: ratio of mass to volume in a substance density = mass volume density remains the same regardless of size or shape of mineral if you cut a mineral in half, you would have half the mass and half the volume so the same density (ratio!)

Special Properties of Minerals Fluorescence & Phosphorescence fluorescence: ability to glow under ultraviolet light phosphorescence: ability to glow after the ultraviolet light is turned off

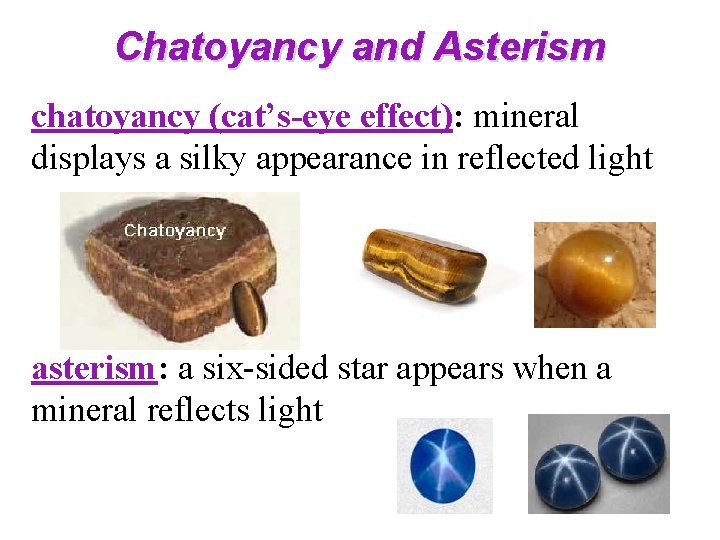
Chatoyancy and Asterism chatoyancy (cat’s-eye effect): mineral displays a silky appearance in reflected light asterism: a six-sided star appears when a mineral reflects light

Double Refraction double refraction: produce a double image of any object viewed through the mineral

Magnetism magnetism: minerals attracted to magnets vthese minerals may be magnetic themselves nonsilicate minerals that contain iron are more likely to be magnetic than silicate minerals Radioactivity radioactivity: unstable atoms (isotopes!) decay by releasing particles & energy to form stable atoms