2 3 Periodic Table Atomic Theory Bohr Models

2. 3 Periodic Table & Atomic Theory
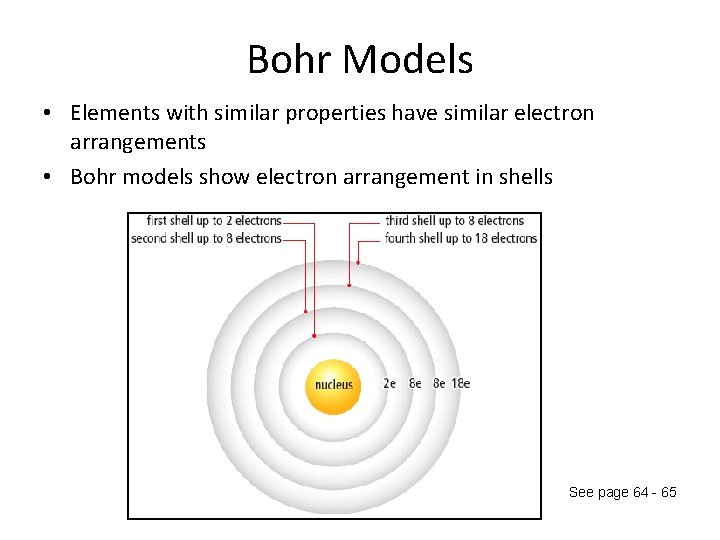
Bohr Models • Elements with similar properties have similar electron arrangements • Bohr models show electron arrangement in shells See page 64 - 65
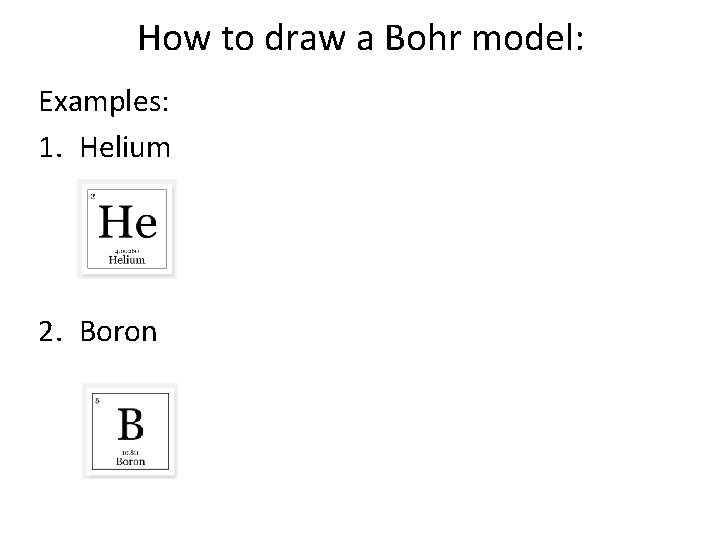
How to draw a Bohr model: Examples: 1. Helium 2. Boron
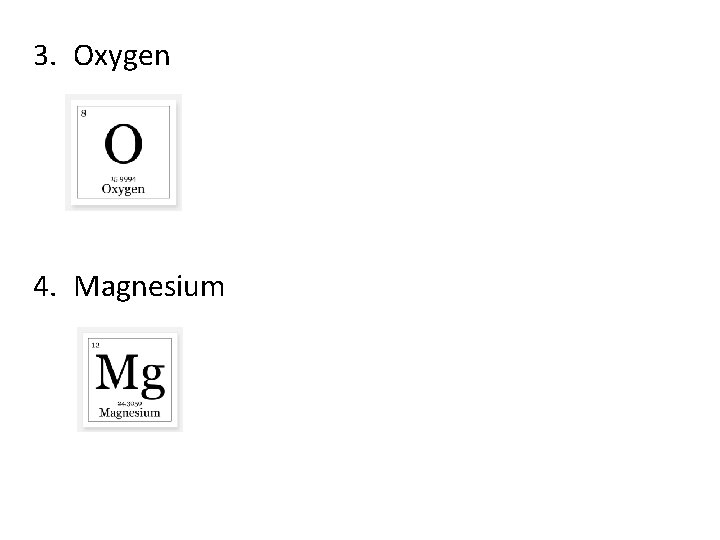
3. Oxygen 4. Magnesium
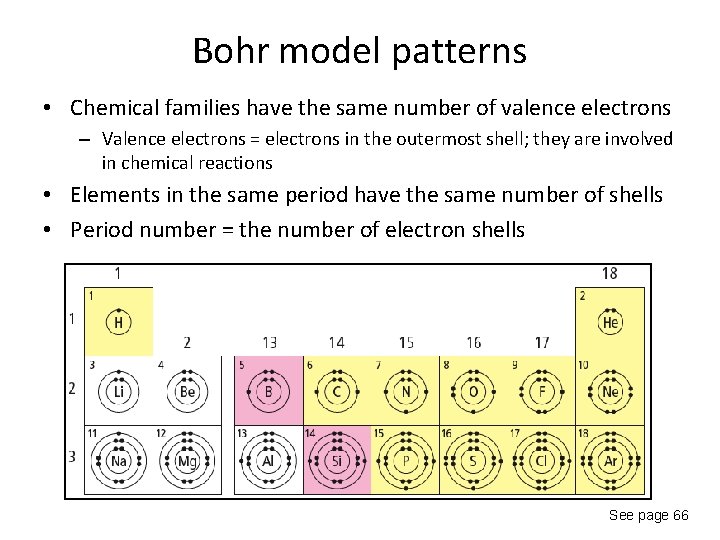
Bohr model patterns • Chemical families have the same number of valence electrons – Valence electrons = electrons in the outermost shell; they are involved in chemical reactions • Elements in the same period have the same number of shells • Period number = the number of electron shells See page 66
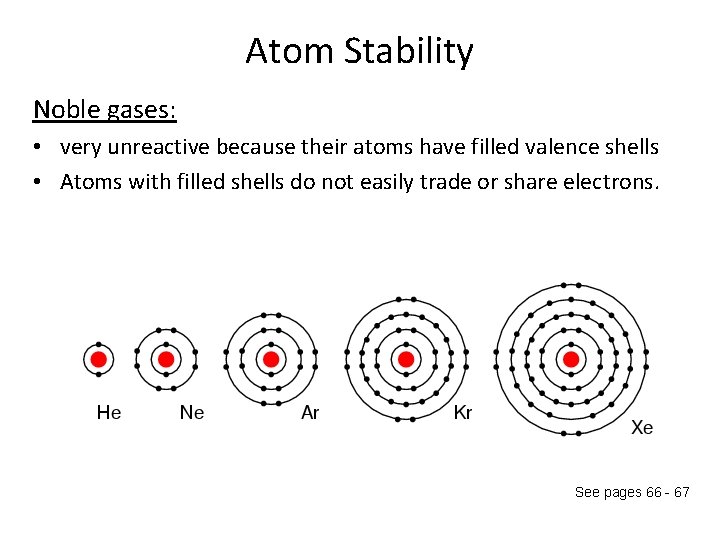
Atom Stability Noble gases: • very unreactive because their atoms have filled valence shells • Atoms with filled shells do not easily trade or share electrons. See pages 66 - 67
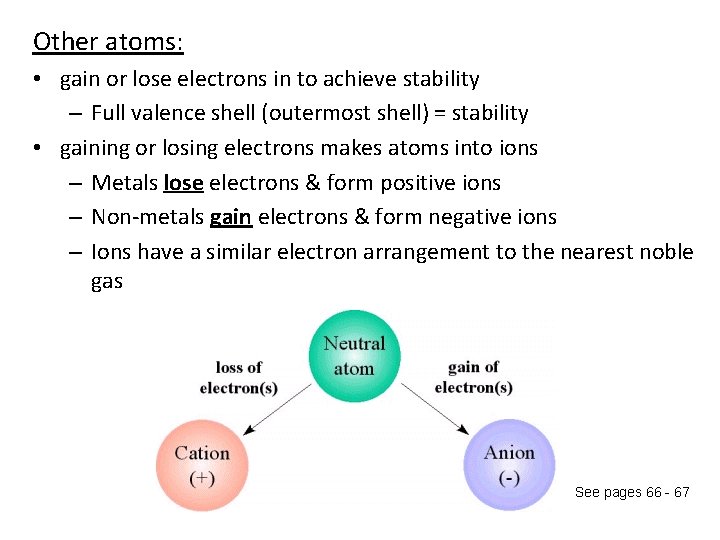
Other atoms: • gain or lose electrons in to achieve stability – Full valence shell (outermost shell) = stability • gaining or losing electrons makes atoms into ions – Metals lose electrons & form positive ions – Non-metals gain electrons & form negative ions – Ions have a similar electron arrangement to the nearest noble gas See pages 66 - 67
Examples of Ion Formation
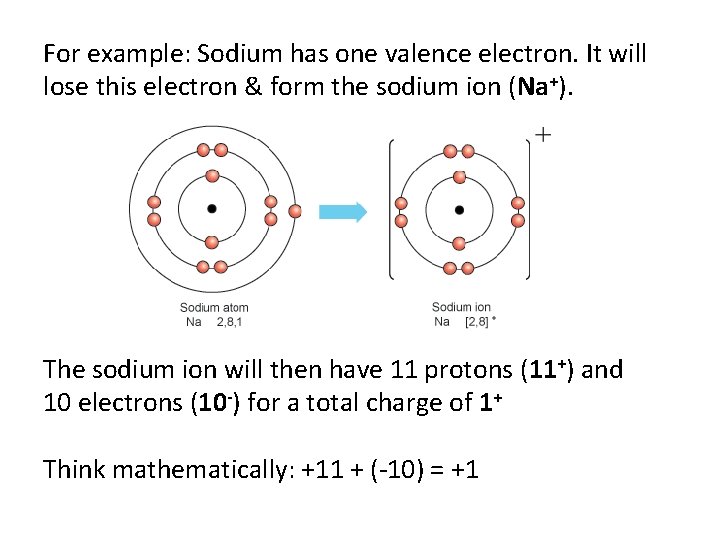
For example: Sodium has one valence electron. It will lose this electron & form the sodium ion (Na+). The sodium ion will then have 11 protons (11+) and 10 electrons (10 -) for a total charge of 1+ Think mathematically: +11 + (-10) = +1
Take the Section 2. 3 Quiz
- Slides: 11