2 3 dehydration dehydration or removal of water






- Slides: 12

2. 3 dehydration • dehydration or removal of water from gas stream is necessary to prevent hydrate formation and increase the heating value of the gas a) water content of gas • f(T, P, composition) • amount gas can “hold” increases with pressure • sour and acid gases can hold more water (increased solubility of water) e. g. 100% C 1 @37. 8 C 500 k. Pa 1000 mg/Sm 3 wet gas 30% C 1 60% CO 2 10% H 2 S 1500 mg/Sm 3 wet gas 100 % CO 2 1700 mg/Sm 3 wet gas - to determine H 2 O content requires experiment/gas analysis

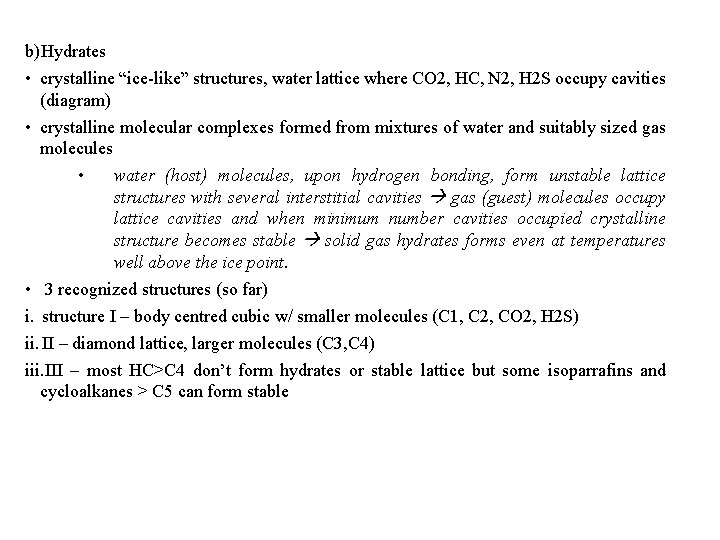
b)Hydrates • crystalline “ice-like” structures, water lattice where CO 2, HC, N 2, H 2 S occupy cavities (diagram) • crystalline molecular complexes formed from mixtures of water and suitably sized gas molecules • water (host) molecules, upon hydrogen bonding, form unstable lattice structures with several interstitial cavities gas (guest) molecules occupy lattice cavities and when minimum number cavities occupied crystalline structure becomes stable solid gas hydrates forms even at temperatures well above the ice point. • 3 recognized structures (so far) i. structure I – body centred cubic w/ smaller molecules (C 1, C 2, CO 2, H 2 S) ii. II – diamond lattice, larger molecules (C 3, C 4) iii. III – most HC>C 4 don’t form hydrates or stable lattice but some isoparrafins and cycloalkanes > C 5 can form stable

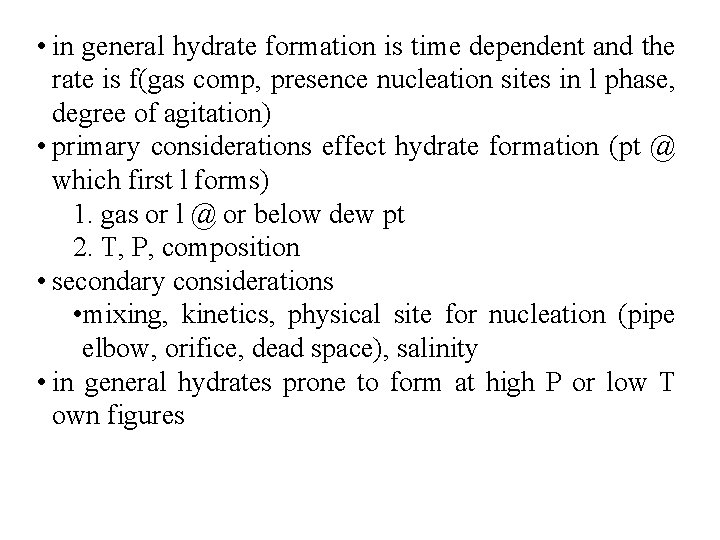
• in general hydrate formation is time dependent and the rate is f(gas comp, presence nucleation sites in l phase, degree of agitation) • primary considerations effect hydrate formation (pt @ which first l forms) 1. gas or l @ or below dew pt 2. T, P, composition • secondary considerations • mixing, kinetics, physical site for nucleation (pipe elbow, orifice, dead space), salinity • in general hydrates prone to form at high P or low T own figures

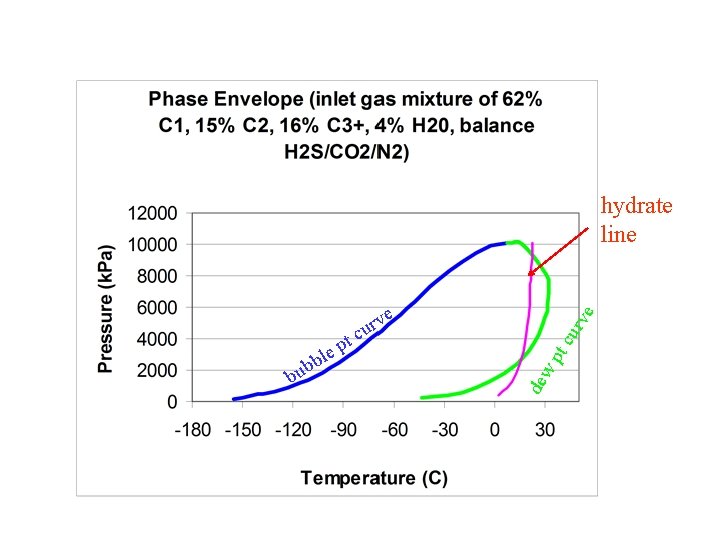
hydrate line e rv u c w pt de b bu cu t p e l b

c) Hydrate inhibition • options to gas dehydration if not practical or feasible try to inhibit the formation of hydrate by adding chemical which shifts the phase diagram away from hydrate (think adding salt to roads) or decrease Thyd form • inject glycols or methanol - combines w/ condensed aqueous phase decreases Thyd form - chemical recovered with aqueous phase at separators d)Gas Dehydration i. glycol units • glycol is a l (DEG, TEG most common, tetraethylene glycol TREG) • applications where TDP depression of 30 -70 C required • usually preceded by inlet gas scrubber to prevent slugging (H 2 O, HC, treatment chem)

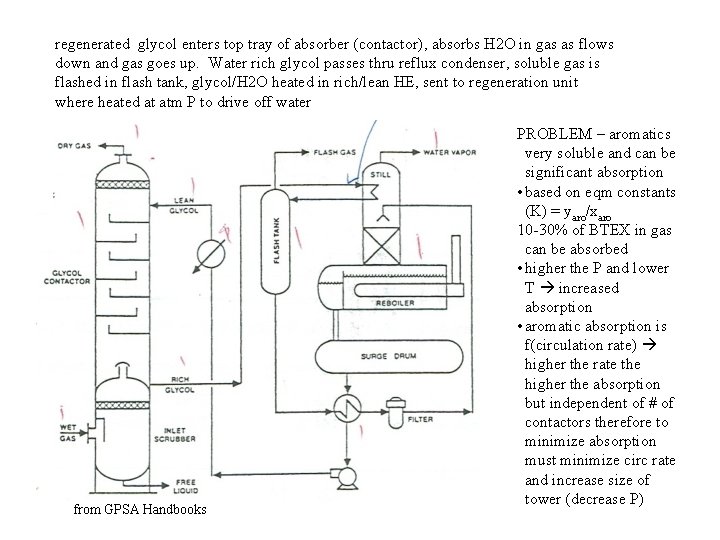
regenerated glycol enters top tray of absorber (contactor), absorbs H 2 O in gas as flows down and gas goes up. Water rich glycol passes thru reflux condenser, soluble gas is flashed in flash tank, glycol/H 2 O heated in rich/lean HE, sent to regeneration unit where heated at atm P to drive off water from GPSA Handbooks PROBLEM – aromatics very soluble and can be significant absorption • based on eqm constants (K) = yaro/xaro 10 -30% of BTEX in gas can be absorbed • higher the P and lower T increased absorption • aromatic absorption is f(circulation rate) higher the rate the higher the absorption but independent of # of contactors therefore to minimize absorption must minimize circ rate and increase size of tower (decrease P)

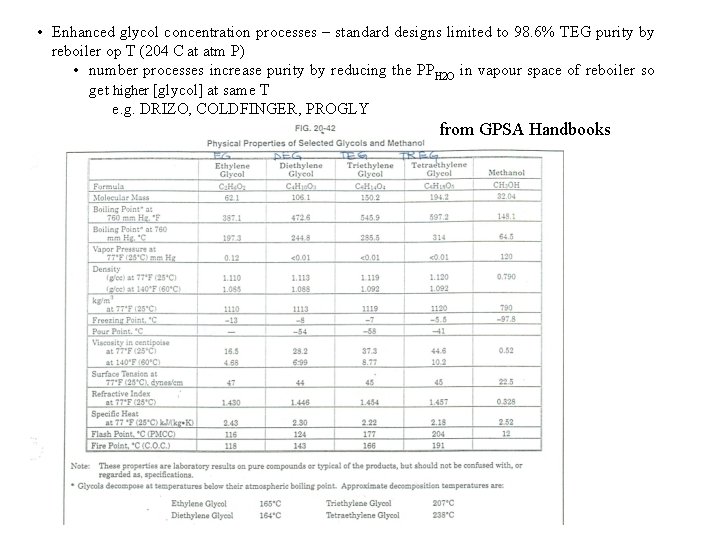
• Enhanced glycol concentration processes – standard designs limited to 98. 6% TEG purity by reboiler op T (204 C at atm P) • number processes increase purity by reducing the PPH 2 O in vapour space of reboiler so get higher [glycol] at same T e. g. DRIZO, COLDFINGER, PROGLY from GPSA Handbooks

• general considerations for glycol units Øif inhibitor present 40 -60% absorbed in glycol which increases duty on reboiler and added volume load Øglycol losses – mechanical carryover from contactor (13 L/106 Sm 3), vapours from contactor/regenerator, foaming in absorber/regen, low P and high T (40 L/106 Sm 3), losses glycol of gas w/ CO 2 is higher than n. gas at P>6200 k. Pa Øbecomes corrosive w/ prolonged exposure to O 2 Ø@ high T (>200 C) decomposition Ølow p. H decomposition

ii. Solid Dehys • comprise of 2 or more towers (one on, one off) - more expensive than glycol units therefore used when: Ø Ø Ø high H 2 S lower dew pt regs simultaneous control of H 2 O and HC dew pt O 2 containing gases where CH 3 OH not favoured both dry/sweeten NGL from Norwegian University of Science and Technology (NTNU)

3 types 1. gels – alumina or silica gels (Si. O 2) v and l dehydrated and HC recovered for natural gas (i. C 5+) hydrocarbon recovery units (HRU), outlet dew pts ~-60 C 2. Alumina – hydrated form Al 2 O 3 (alumina oxide), TDP~-70 C, less heat required than mol sieve and Tregnerator lower 3. molecular sieve – aluminosilicates, high H 2 O capacity and produces lowest TDP ~-100 C , can sweeten and dry gases and liquids (fig 2069) • H 2 O capacity less dependent on ambient T and relative humidity • expensive • commonly used ahead NGL plants to recover C 2 from GPSA Handbooks

iii. Membranes • separate gas from H 2 O, CO 2, HC according to permeability where dissolve/diffuse through membrane • driving force is differential PP across membrane • CO 2/H 2 O permeate thru membrane permeate at reduced P while nonpermeate @ P slightly<Pfeed • C 1+ in permeate f(∆P, SA membrane), 5 -10% carryover • only applicable to plants use low P natural gas fuels

e)Dehydration of Liquid Phase HC • typically amount of water in HC l is low, even at saturation (Fig 20 -2) i. gas stripper • counter current stripper w/ dry gas, used offshore, trayed contactor and stripper • low cost, simple • need dry n. gas stream, waste stream of VHC from condensate ii. solid desiccant • activated alumina, Tdp~-70 C, absorbs heavy HC • Ca. Cl 2 – brine has neg effect • MS too expensive for H 2 O removal iii. distillation • fractionation columns for use in dehy of NGLs • higher energy requirements
 Water and water and water water
Water and water and water water Removal of water hardness
Removal of water hardness Symtons of dehydration
Symtons of dehydration Severe dehydration
Severe dehydration Severe dehydration
Severe dehydration Dehydration in microtechnique
Dehydration in microtechnique Dehydration types
Dehydration types Dehydration vs hydrolysis
Dehydration vs hydrolysis Dehydration of 2-methylcyclohexanol
Dehydration of 2-methylcyclohexanol Dehydration synethesis
Dehydration synethesis Dehydration synthesis of amino acids
Dehydration synthesis of amino acids Symtons of dehydration
Symtons of dehydration Iv fluid computation
Iv fluid computation