2 3 CarbonBased Molecules KEY CONCEPT Carbonbased molecules
2. 3 Carbon-Based Molecules KEY CONCEPT Carbon-based molecules are the foundation of life.

2. 3 Carbon-Based Molecules Carbon atoms have unique bonding properties. • Carbon forms covalent bonds with up to four other atoms, including other carbon atoms.
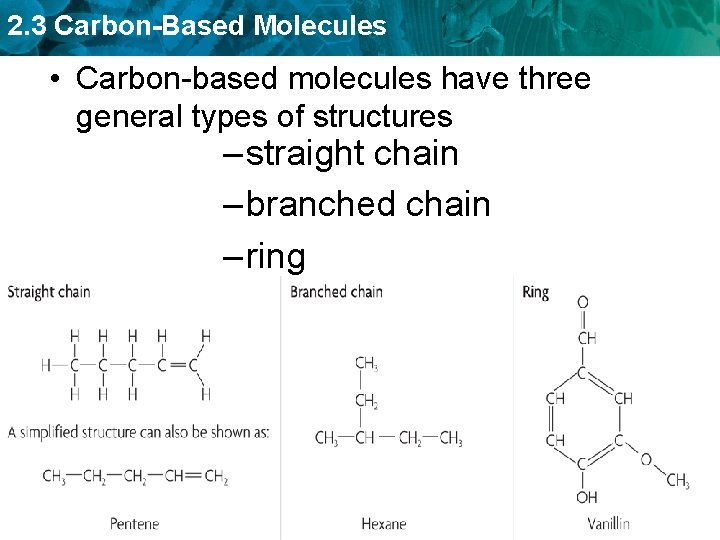
2. 3 Carbon-Based Molecules • Carbon-based molecules have three general types of structures – straight chain – branched chain – ring

2. 3 Carbon-Based Molecules • Many carbon-based molecules are made of many small subunits bonded together. – Monomers are the individual subunits. – Polymers are made of many monomers.
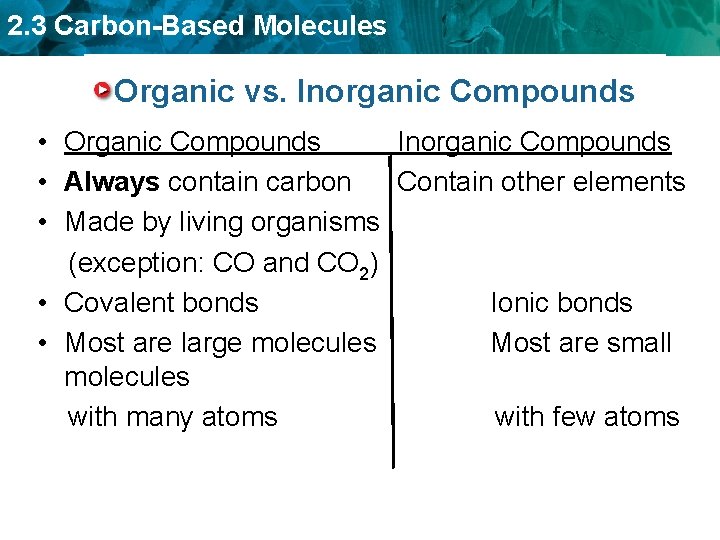
2. 3 Carbon-Based Molecules Organic vs. Inorganic Compounds • Organic Compounds Inorganic Compounds • Always contain carbon Contain other elements • Made by living organisms (exception: CO and CO 2) • Covalent bonds Ionic bonds • Most are large molecules Most are small molecules with many atoms with few atoms
2. 3 Carbon-Based Molecules Four main types of carbon-based molecules are found in living things. • Carbohydrates • Lipids • Protein • Nucleic acid

2. 3 Carbon-Based Molecules Carbohydrates are made of carbon, hydrogen, and oxygen. There are exactly twice as many Hydrogen as Oxygen
2. 3 Carbon-Based Molecules – Carbohydrates include sugars and starches. – Monomer. Monosaccharides are simple sugars. – Polymer. Polysaccharides include starches, cellulose, and glycogen.
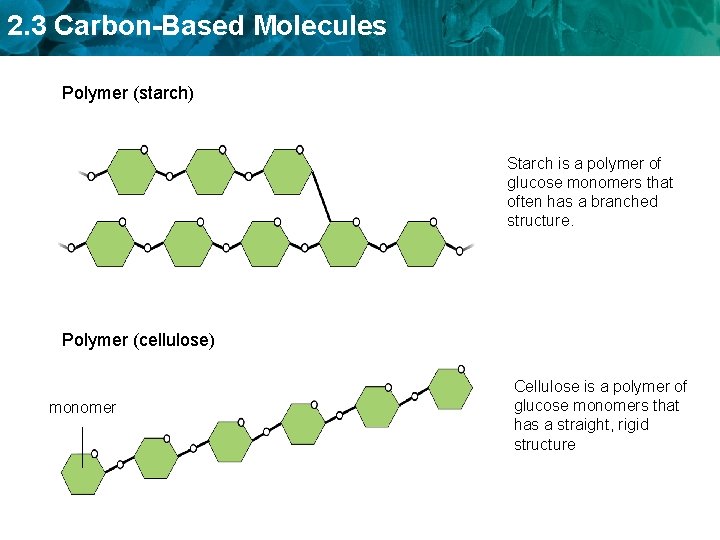
2. 3 Carbon-Based Molecules Polymer (starch) Starch is a polymer of glucose monomers that often has a branched structure. Polymer (cellulose) monomer Cellulose is a polymer of glucose monomers that has a straight, rigid structure
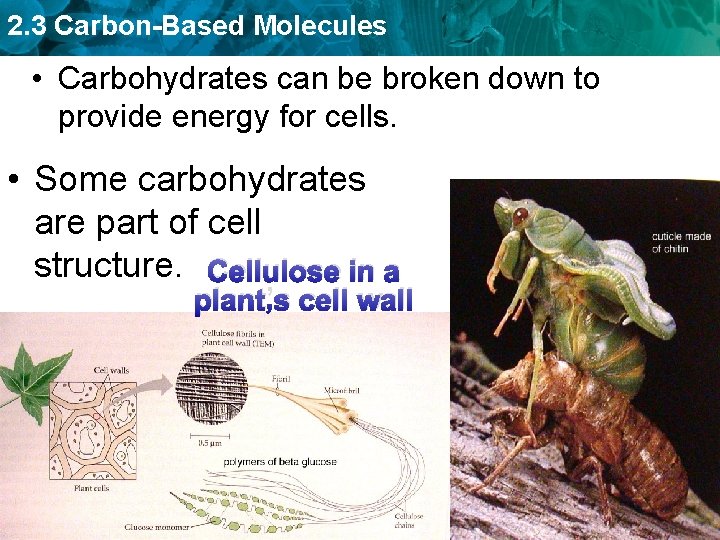
2. 3 Carbon-Based Molecules • Carbohydrates can be broken down to provide energy for cells. • Some carbohydrates are part of cell structure. Cellulose in a plant’s cell wall
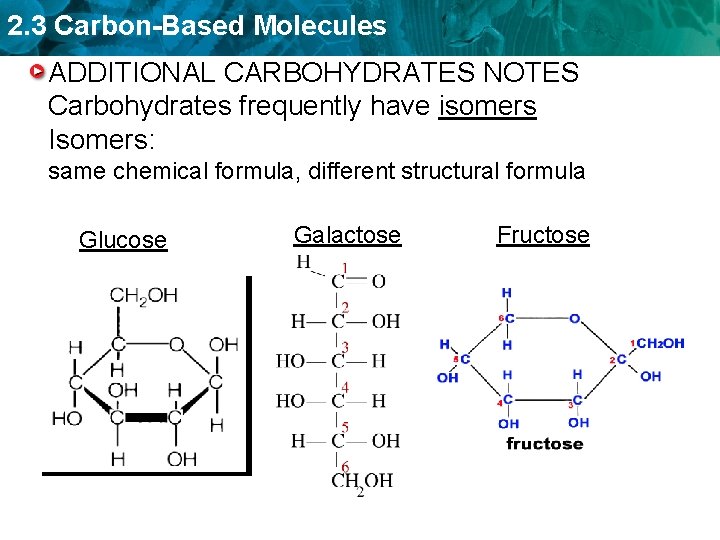
2. 3 Carbon-Based Molecules ADDITIONAL CARBOHYDRATES NOTES Carbohydrates frequently have isomers Isomers: same chemical formula, different structural formula Glucose Galactose Fructose
2. 3 Carbon-Based Molecules • Lipids are nonpolar molecules that include fats, oils, and cholesterol. – Lipids contain carbon, hydrogen & oxygen – There always more than twice as many hydrogen as oxygen in lipids. – Many contain carbon chains called fatty acids. – Fats and oils contain fatty acids bonded to glycerol. – Monomers: fatty acids and glycerol Triglyceride
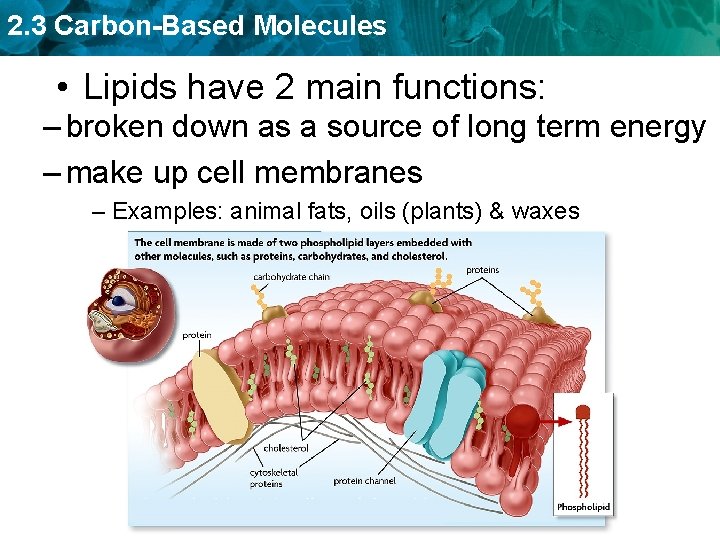
2. 3 Carbon-Based Molecules • Lipids have 2 main functions: – broken down as a source of long term energy – make up cell membranes – Examples: animal fats, oils (plants) & waxes
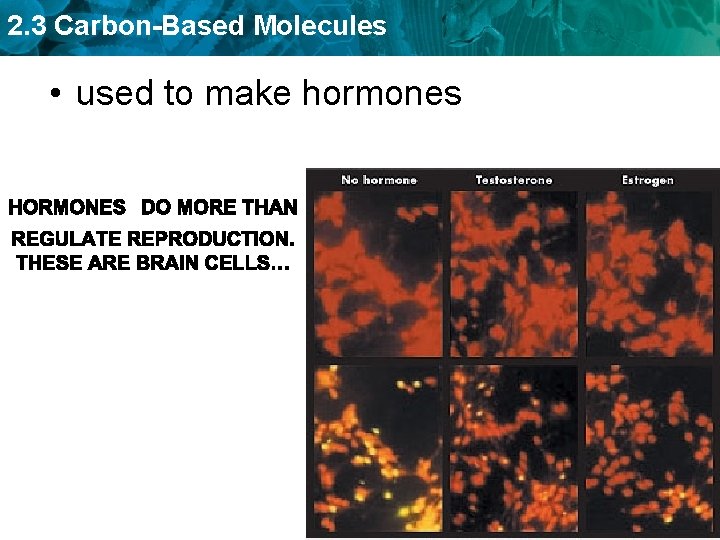
2. 3 Carbon-Based Molecules • used to make hormones

2. 3 Carbon-Based Molecules Fats & Oils (Additional Notes on Lipids) • Fats & Oils are a major category of lipids.
2. 3 Carbon-Based Molecules Types of Fats & Oils • There are 3 main types: 1. Saturated Fats 2. Unsaturated Fats 3. Hydrogenated Fats
2. 3 Carbon-Based Molecules • Saturated Fats Generally animal fats Examples: butter, shortening, lard Solid at room temperature Full of Hydrogen atoms
2. 3 Carbon-Based Molecules Unsaturated Fats • Generally found in plants – Examples: - olive oil, corn oil, sunflower oil, peanut oil • Liquid at room temperature • Missing Hydrogen atoms – [Causes double bonds between C atoms]
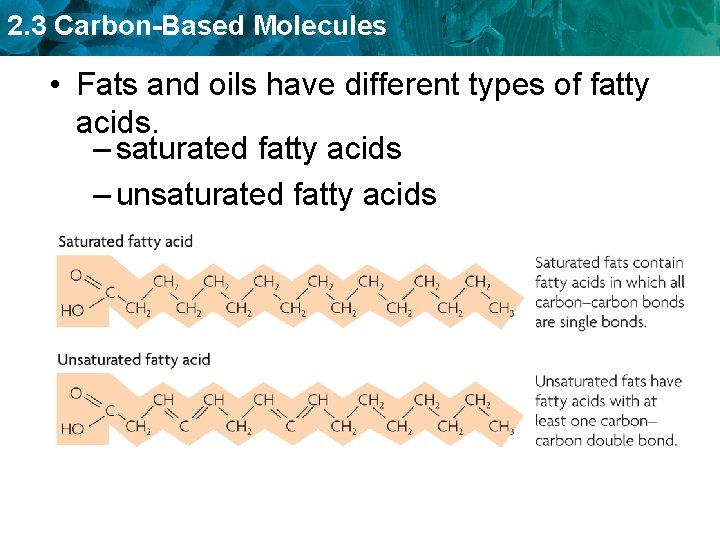
2. 3 Carbon-Based Molecules • Fats and oils have different types of fatty acids. – saturated fatty acids – unsaturated fatty acids
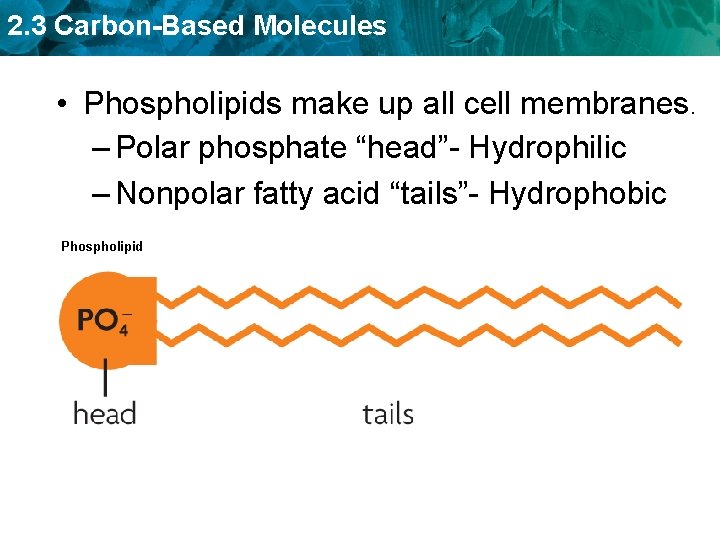
2. 3 Carbon-Based Molecules • Phospholipids make up all cell membranes. – Polar phosphate “head”- Hydrophilic – Nonpolar fatty acid “tails”- Hydrophobic Phospholipid

2. 3 Carbon-Based Molecules • Proteins are polymers of amino acid monomers. Proteins contain carbon, hydrogen, oxygen, and Nitrogen. Monomer: Amino Acid • Twenty different amino acids are used to build proteins in organisms. • Amino acids differ in R groups.
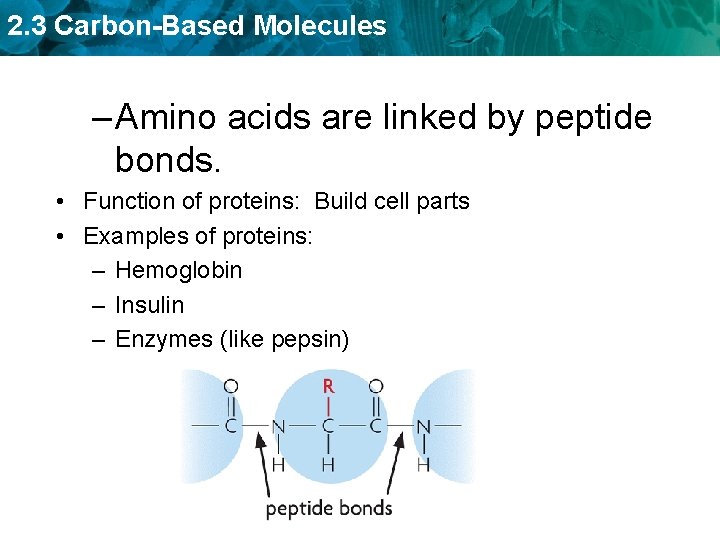
2. 3 Carbon-Based Molecules – Amino acids are linked by peptide bonds. • Function of proteins: Build cell parts • Examples of proteins: – Hemoglobin – Insulin – Enzymes (like pepsin)
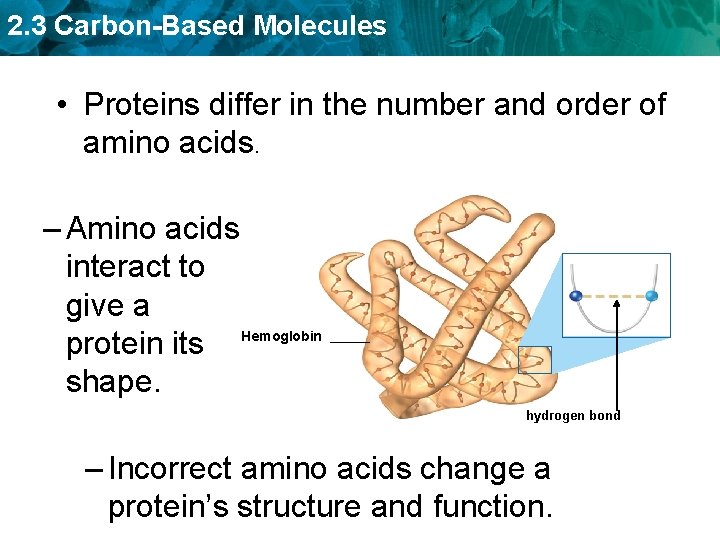
2. 3 Carbon-Based Molecules • Proteins differ in the number and order of amino acids. – Amino acids interact to give a protein its Hemoglobin shape. hydrogen bond – Incorrect amino acids change a protein’s structure and function.
2. 3 Carbon-Based Molecules Additional Protein Notes Proteins are strings of amino acids. The number and order of amino acids makes each enzyme different • Enzymes are a special kind of protein • Enzymes speed up processes in living things. • They help break things down (like in digestion) • They help build things (like cell parts)
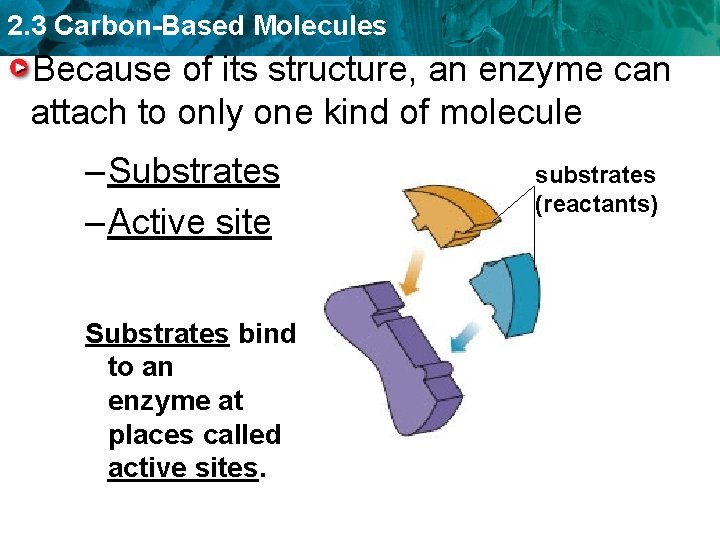
2. 3 Carbon-Based Molecules Because of its structure, an enzyme can attach to only one kind of molecule – Substrates – Active site Substrates bind to an enzyme at places called active sites. substrates (reactants)
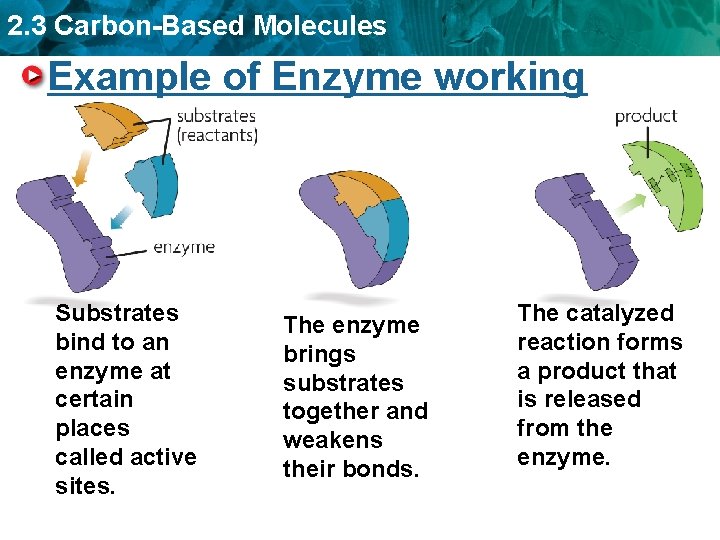
2. 3 Carbon-Based Molecules Example of Enzyme working Substrates bind to an enzyme at certain places called active sites. The enzyme brings substrates together and weakens their bonds. The catalyzed reaction forms a product that is released from the enzyme.
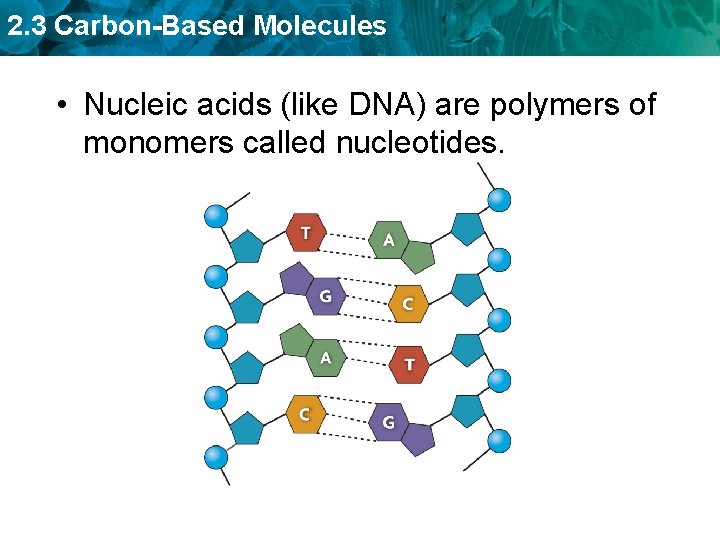
2. 3 Carbon-Based Molecules • Nucleic acids (like DNA) are polymers of monomers called nucleotides.
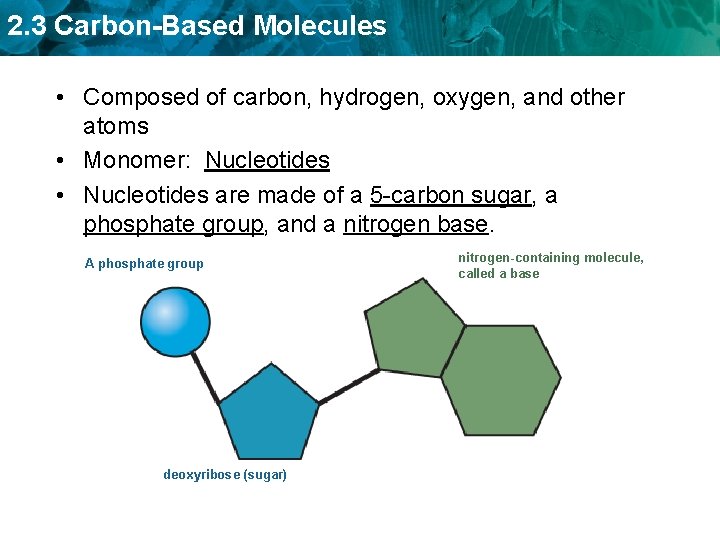
2. 3 Carbon-Based Molecules • Composed of carbon, hydrogen, oxygen, and other atoms • Monomer: Nucleotides • Nucleotides are made of a 5 -carbon sugar, a phosphate group, and a nitrogen base. A phosphate group deoxyribose (sugar) nitrogen-containing molecule, called a base
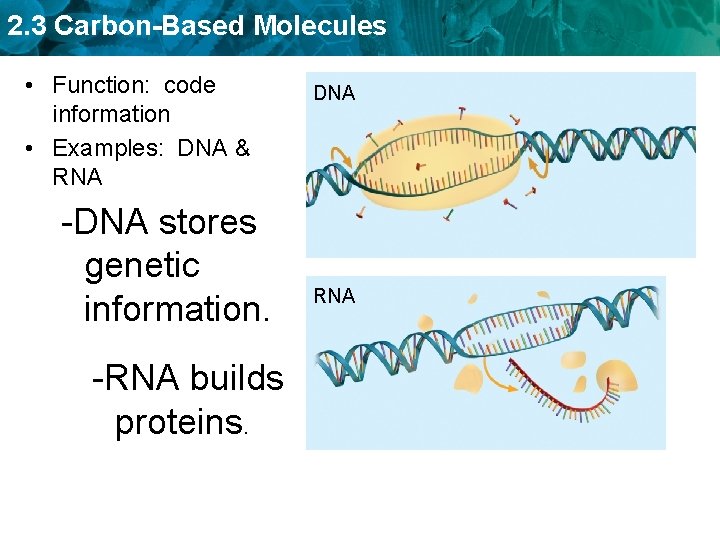
2. 3 Carbon-Based Molecules • Function: code information • Examples: DNA & RNA -DNA stores genetic information. -RNA builds proteins. DNA RNA
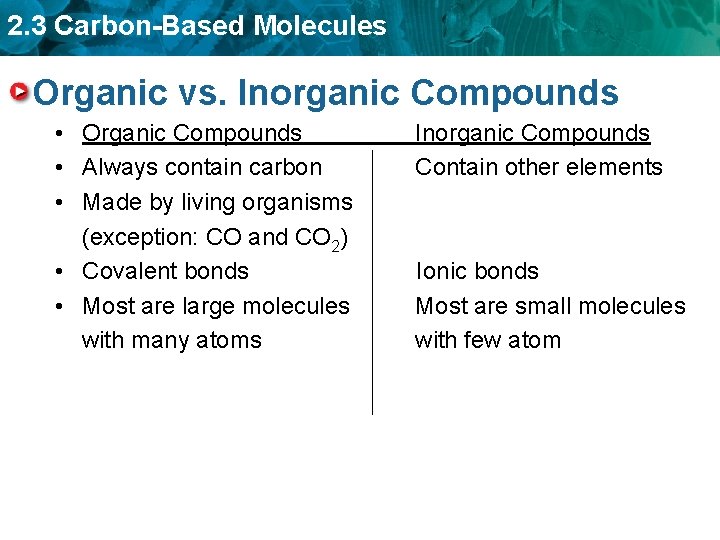
2. 3 Carbon-Based Molecules Organic vs. Inorganic Compounds • Organic Compounds • Always contain carbon • Made by living organisms (exception: CO and CO 2) • Covalent bonds • Most are large molecules with many atoms Inorganic Compounds Contain other elements Ionic bonds Most are small molecules with few atom
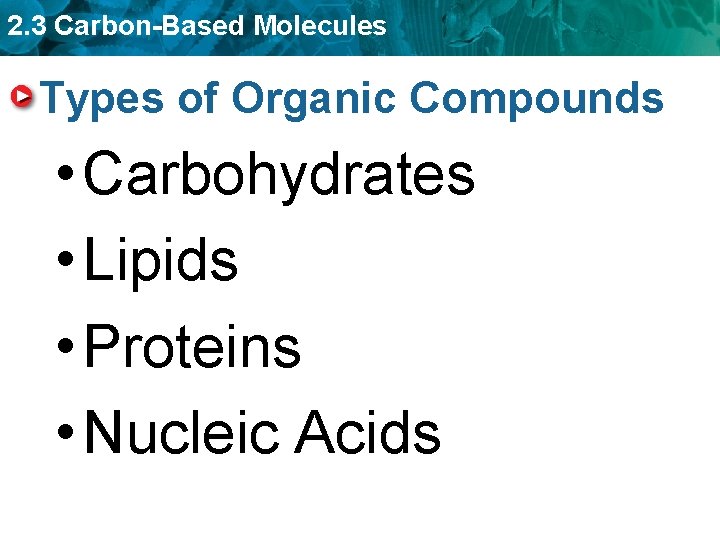
2. 3 Carbon-Based Molecules Types of Organic Compounds • Carbohydrates • Lipids • Proteins • Nucleic Acids
- Slides: 31