2 2 Measured Numbers and Significant Figures Length
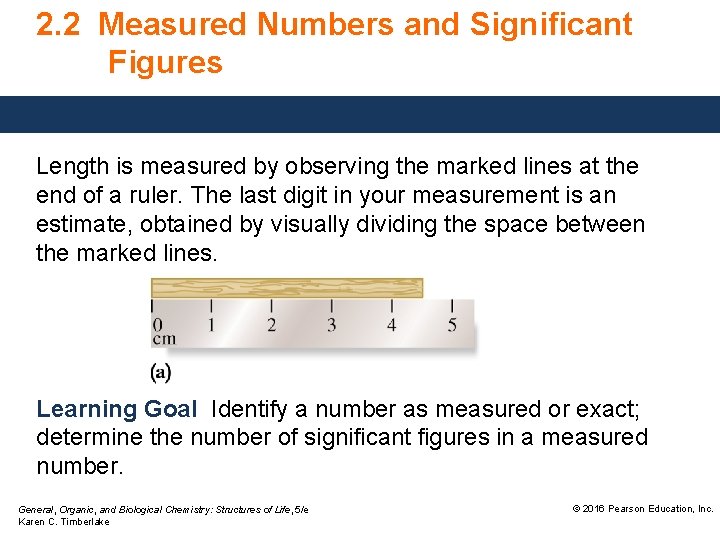
2. 2 Measured Numbers and Significant Figures Length is measured by observing the marked lines at the end of a ruler. The last digit in your measurement is an estimate, obtained by visually dividing the space between the marked lines. Learning Goal Identify a number as measured or exact; determine the number of significant figures in a measured number. General, Organic, and Biological Chemistry: Structures of Life, 5/e Karen C. Timberlake © 2016 Pearson Education, Inc.
Measured Numbers Measured numbers are the numbers obtained when you measure a quantity such as your height, weight, or temperature. To write a measured number, • observe the numerical values of the marked lines. • estimate the value of the number between the marks. The estimated number is the final number in your measured number. General, Organic, and Biological Chemistry: Structures of Life, 5/e Karen C. Timberlake © 2016 Pearson Education, Inc.
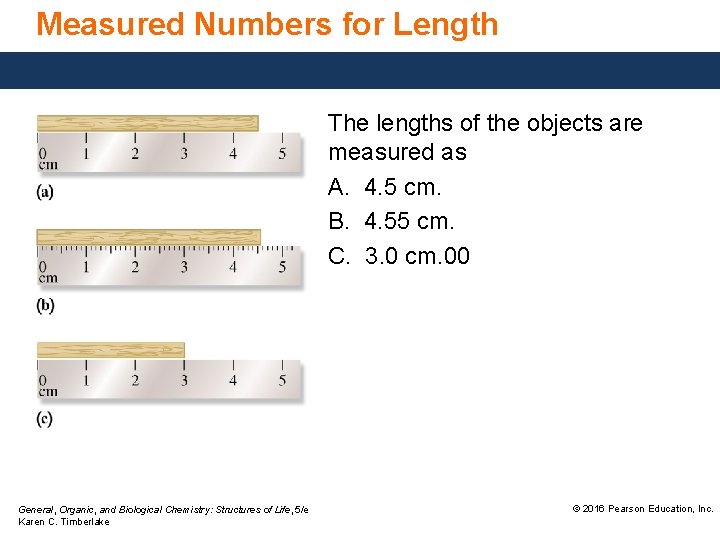
Measured Numbers for Length The lengths of the objects are measured as A. 4. 5 cm. B. 4. 55 cm. C. 3. 0 cm. 00 General, Organic, and Biological Chemistry: Structures of Life, 5/e Karen C. Timberlake © 2016 Pearson Education, Inc.
Significant Figures In a measured number, the significant figures (SFs) are all the digits, including the estimated digit. Significant figures • are used to represent the amount of error associated with a measurement. • are all nonzero digits and zeros between digits. • are not zeros that act as placeholders before digits. • are zeros at the end of a decimal number. Core Chemistry Skill Counting Significant Figures General, Organic, and Biological Chemistry: Structures of Life, 5/e Karen C. Timberlake © 2016 Pearson Education, Inc.
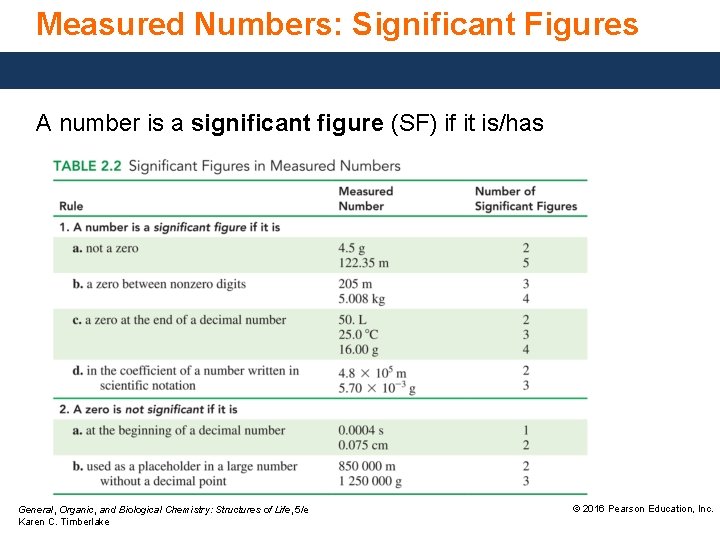
Measured Numbers: Significant Figures A number is a significant figure (SF) if it is/has General, Organic, and Biological Chemistry: Structures of Life, 5/e Karen C. Timberlake © 2016 Pearson Education, Inc.
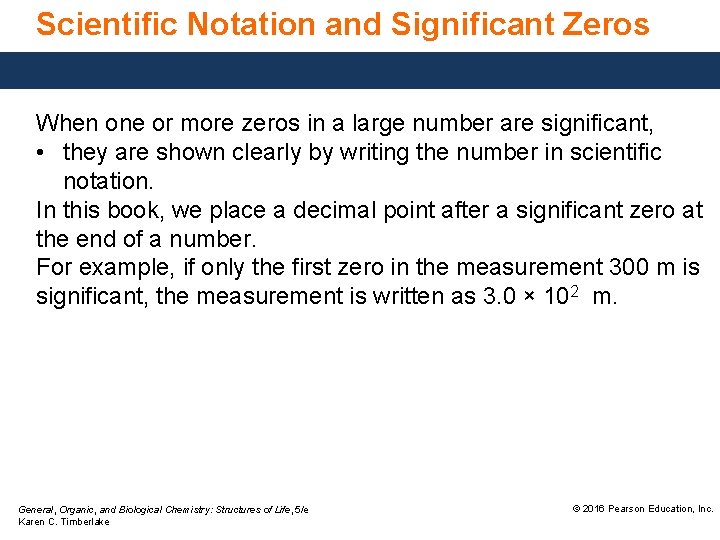
Scientific Notation and Significant Zeros When one or more zeros in a large number are significant, • they are shown clearly by writing the number in scientific notation. In this book, we place a decimal point after a significant zero at the end of a number. For example, if only the first zero in the measurement 300 m is significant, the measurement is written as 3. 0 × 102 m. General, Organic, and Biological Chemistry: Structures of Life, 5/e Karen C. Timberlake © 2016 Pearson Education, Inc.
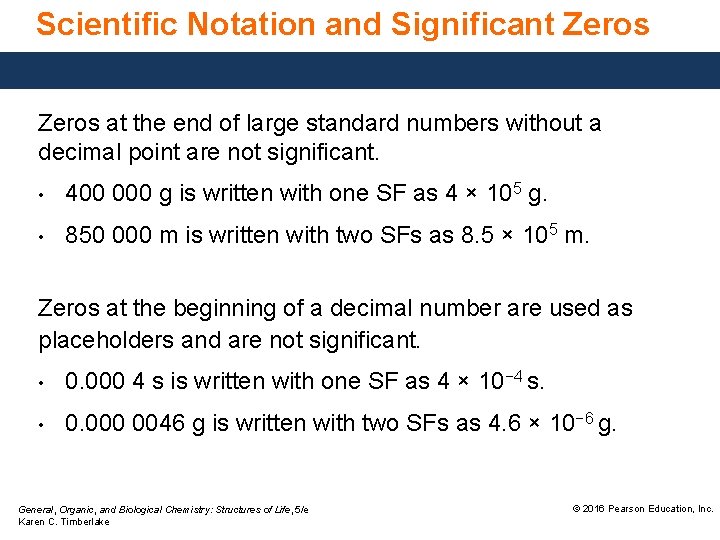
Scientific Notation and Significant Zeros at the end of large standard numbers without a decimal point are not significant. • 400 000 g is written with one SF as 4 × 105 g. • 850 000 m is written with two SFs as 8. 5 × 105 m. Zeros at the beginning of a decimal number are used as placeholders and are not significant. • 0. 000 4 s is written with one SF as 4 × 10− 4 s. • 0. 000 0046 g is written with two SFs as 4. 6 × 10− 6 g. General, Organic, and Biological Chemistry: Structures of Life, 5/e Karen C. Timberlake © 2016 Pearson Education, Inc.
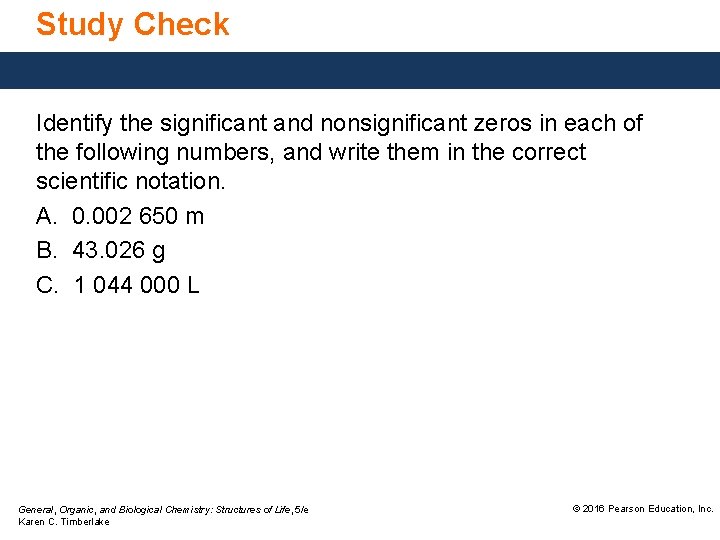
Study Check Identify the significant and nonsignificant zeros in each of the following numbers, and write them in the correct scientific notation. A. 0. 002 650 m B. 43. 026 g C. 1 044 000 L General, Organic, and Biological Chemistry: Structures of Life, 5/e Karen C. Timberlake © 2016 Pearson Education, Inc.
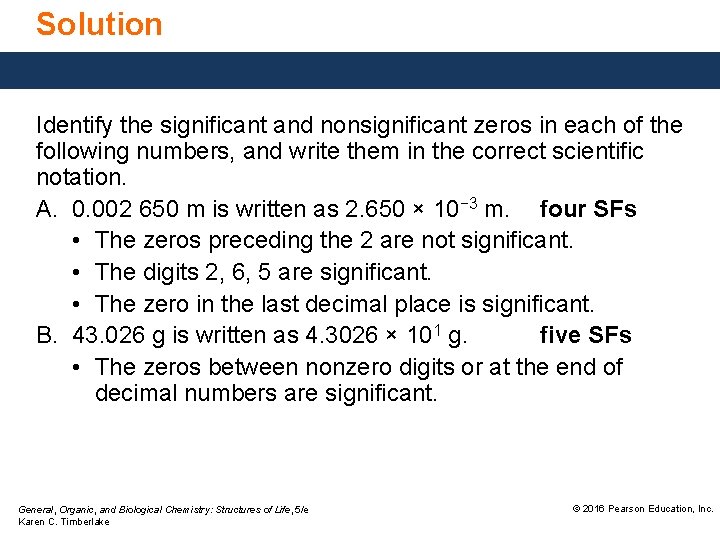
Solution Identify the significant and nonsignificant zeros in each of the following numbers, and write them in the correct scientific notation. A. 0. 002 650 m is written as 2. 650 × 10− 3 m. four SFs • The zeros preceding the 2 are not significant. • The digits 2, 6, 5 are significant. • The zero in the last decimal place is significant. B. 43. 026 g is written as 4. 3026 × 101 g. five SFs • The zeros between nonzero digits or at the end of decimal numbers are significant. General, Organic, and Biological Chemistry: Structures of Life, 5/e Karen C. Timberlake © 2016 Pearson Education, Inc.
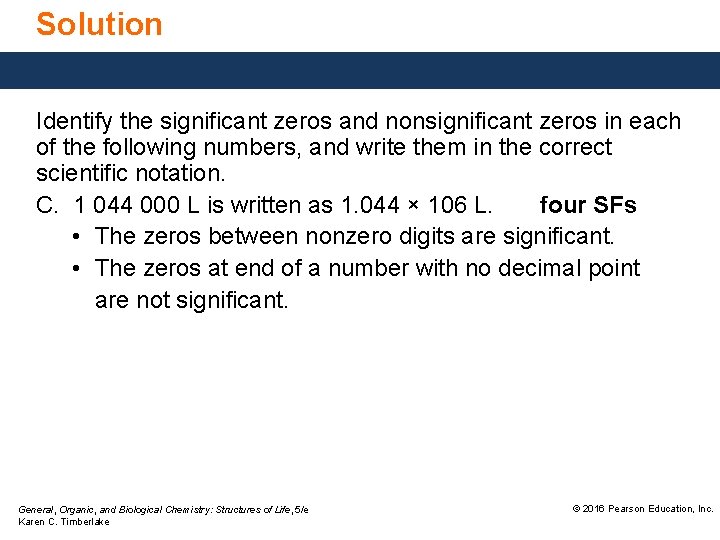
Solution Identify the significant zeros and nonsignificant zeros in each of the following numbers, and write them in the correct scientific notation. C. 1 044 000 L is written as 1. 044 × 106 L. four SFs • The zeros between nonzero digits are significant. • The zeros at end of a number with no decimal point are not significant. General, Organic, and Biological Chemistry: Structures of Life, 5/e Karen C. Timberlake © 2016 Pearson Education, Inc.
Exact Numbers Exact numbers are • not measured and do not have a limited number of significant figures. • not used to find the number of significant figures in a calculated answer. • numbers obtained by counting. 8 cookies • definitions that compare two units. 6 eggs • definitions in the same measuring system. 1 qt = 4 cups 1 kg = 1 000 g General, Organic, and Biological Chemistry: Structures of Life, 5/e Karen C. Timberlake © 2016 Pearson Education, Inc.
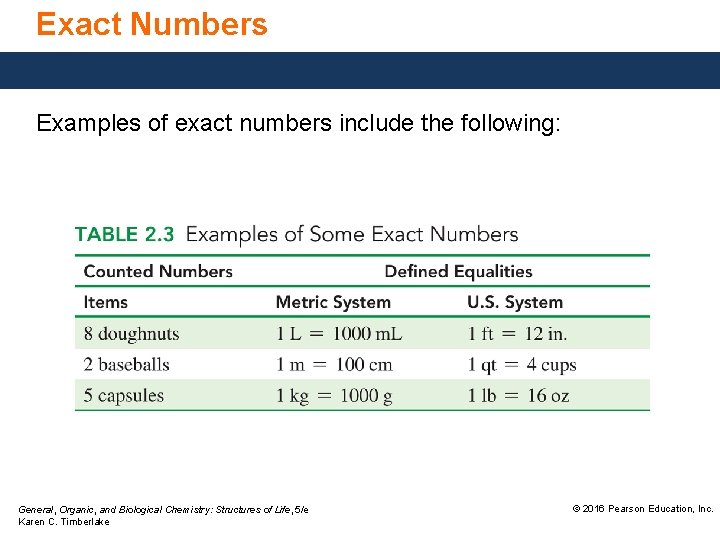
Exact Numbers Examples of exact numbers include the following: General, Organic, and Biological Chemistry: Structures of Life, 5/e Karen C. Timberlake © 2016 Pearson Education, Inc.
Study Check Identify the numbers below as measured or exact, and give the number of significant figures in each measured number. A. 3 coins B. The diameter of a circle is 7. 902 cm. C. 60 min = 1 h General, Organic, and Biological Chemistry: Structures of Life, 5/e Karen C. Timberlake © 2016 Pearson Education, Inc.
Solution Identify the numbers below as measured or exact, and give the number of significant figures in each measured number. A. 3 coins is a counting number and, therefore, is an exact number. B. The diameter of a circle is 7. 902 cm. This is a measured number and the zero is significant, so it contains four SF. C. The relationship 60 min = 1 h is exact by definition. General, Organic, and Biological Chemistry: Structures of Life, 5/e Karen C. Timberlake © 2016 Pearson Education, Inc.
- Slides: 14