2 2 Balancing Chemical Equations In a chemical

2 -2 Balancing Chemical Equations

• In a chemical change the atoms and ions are rearranged to form new compounds with new properties. • A chemical reaction occurs when one or more chemical changes happen at the same time. • The original compounds are called reactants • The new compounds that are made are called products • Chemical reactions are represented by chemical equations.
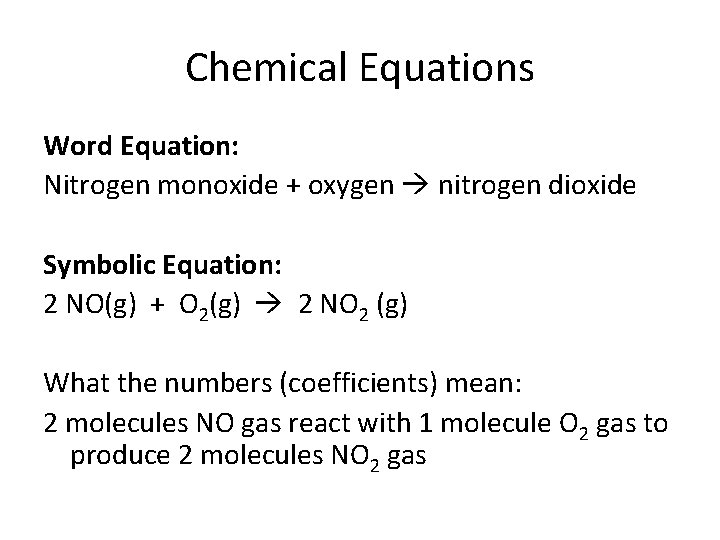
Chemical Equations Word Equation: Nitrogen monoxide + oxygen nitrogen dioxide Symbolic Equation: 2 NO(g) + O 2(g) 2 NO 2 (g) What the numbers (coefficients) mean: 2 molecules NO gas react with 1 molecule O 2 gas to produce 2 molecules NO 2 gas
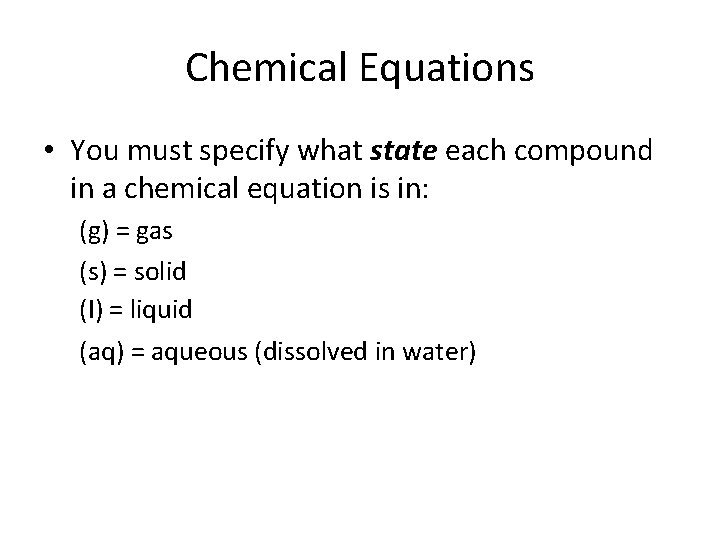
Chemical Equations • You must specify what state each compound in a chemical equation is in: (g) = gas (s) = solid (l) = liquid (aq) = aqueous (dissolved in water)
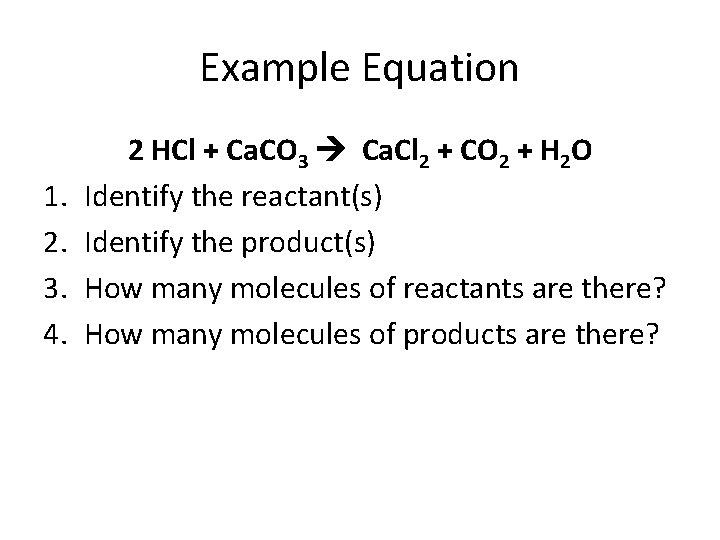
Example Equation 1. 2. 3. 4. 2 HCl + Ca. CO 3 Ca. Cl 2 + CO 2 + H 2 O Identify the reactant(s) Identify the product(s) How many molecules of reactants are there? How many molecules of products are there?

Law of Conservation of Mass • The total mass of the reactants MUST equal the total mass of the products • This is why equations must be BALANCED!
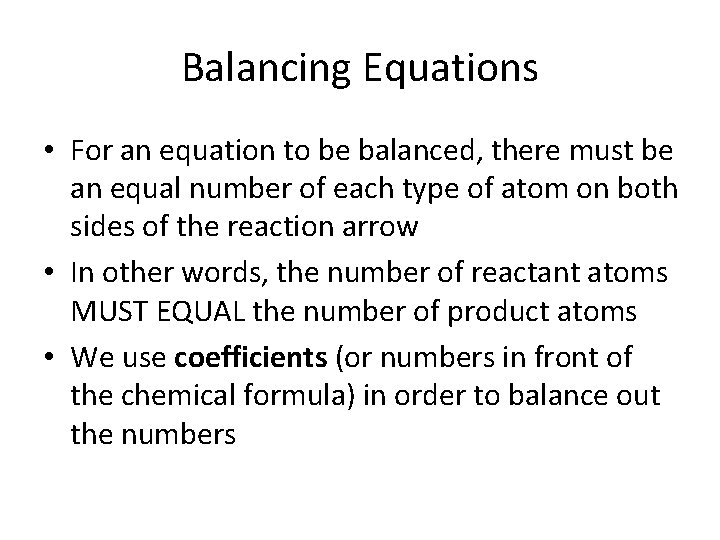
Balancing Equations • For an equation to be balanced, there must be an equal number of each type of atom on both sides of the reaction arrow • In other words, the number of reactant atoms MUST EQUAL the number of product atoms • We use coefficients (or numbers in front of the chemical formula) in order to balance out the numbers
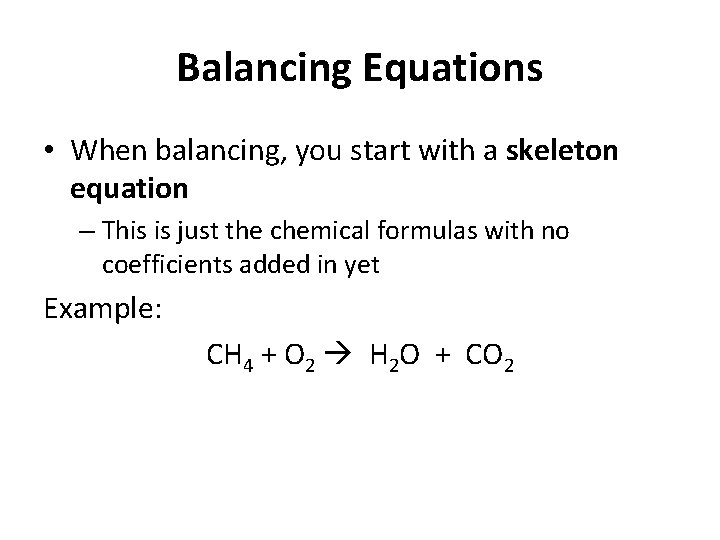
Balancing Equations • When balancing, you start with a skeleton equation – This is just the chemical formulas with no coefficients added in yet Example: CH 4 + O 2 H 2 O + CO 2
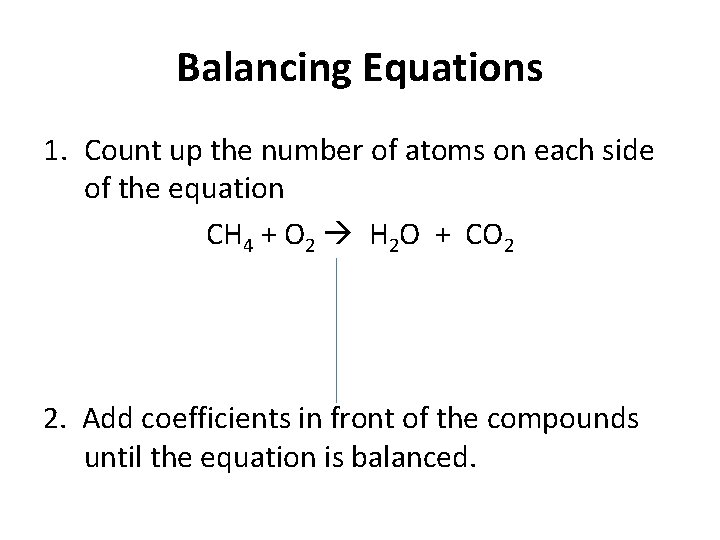
Balancing Equations 1. Count up the number of atoms on each side of the equation CH 4 + O 2 H 2 O + CO 2 2. Add coefficients in front of the compounds until the equation is balanced.

Balancing Equations • Order for balancing: 1. 2. 3. 4. Metals Non-Metals Oxygen Hydrogen • You don’t HAVE to balance in this order, but I find it makes it easiest!
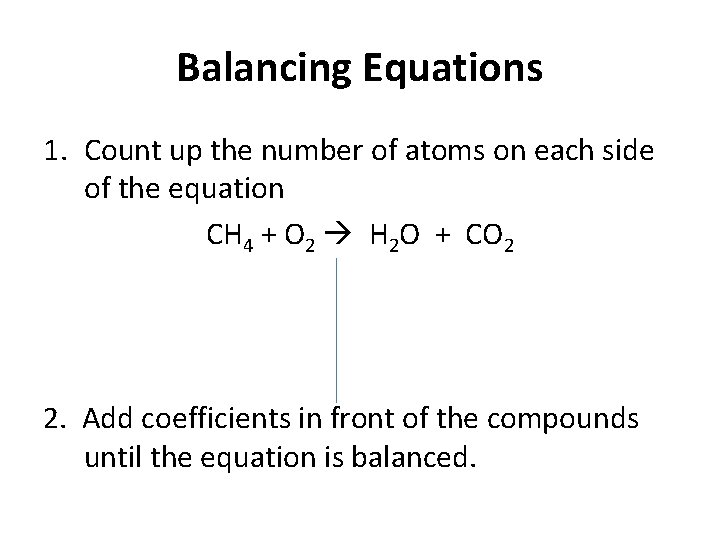
Balancing Equations 1. Count up the number of atoms on each side of the equation CH 4 + O 2 H 2 O + CO 2 2. Add coefficients in front of the compounds until the equation is balanced.
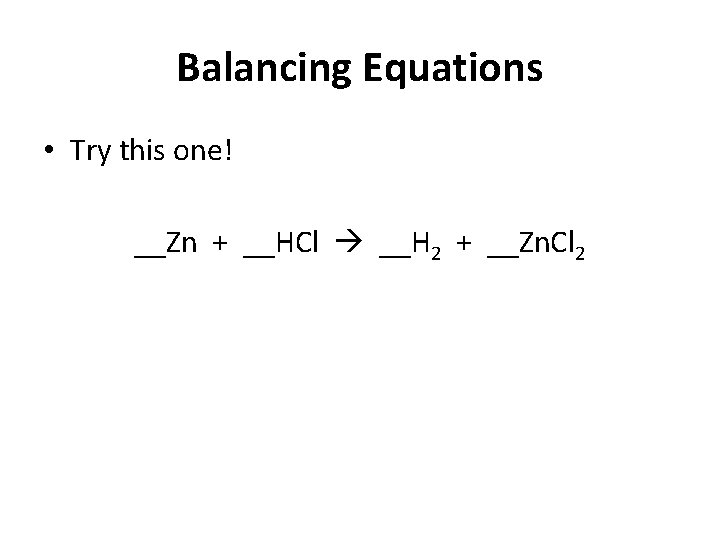
Balancing Equations • Try this one! __Zn + __HCl __H 2 + __Zn. Cl 2
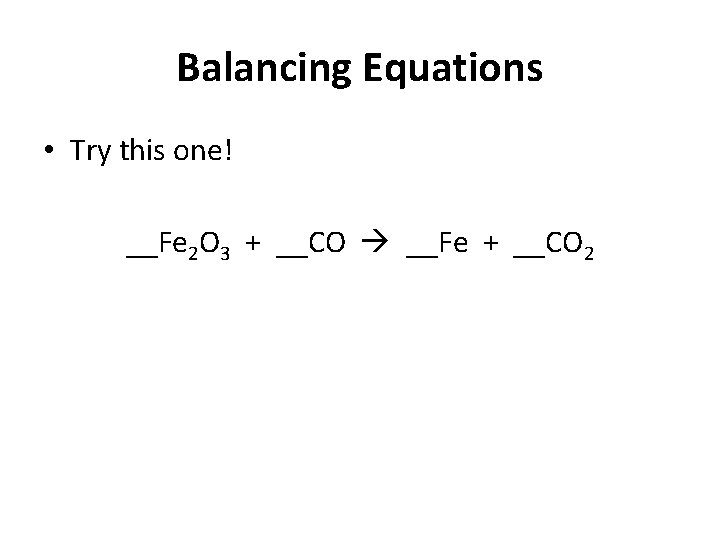
Balancing Equations • Try this one! __Fe 2 O 3 + __CO __Fe + __CO 2

Balancing Equations • Assignment: 1. Practice problems page 211 2. Start working through the big balancing equations worksheet booklet!
- Slides: 14