2 2 2 Electron energy loss spectroscopy EELS




- Slides: 21

2. 2. 2. Electron energy loss spectroscopy (EELS) Primary Inelastically scattered electrons, which have lost well–defined energies in the course of interaction with the solid surface, are considered in EELS. The change in electron energy is not random but is directly related to # which electron # from which atom # from which orbital shell the inelastic colision took palce. This specific loos of energy is known as EELS. These losses cover an energy range from: 10 -3 to 104 e. V and originate from different scattering processes:

100 -3 – 1 1 – 100 Exitation of vibrations of surface atoms and adsorbates Exitation of plasmons and electronic interband transitions high-resolution EELS conventional EELS Exitation source with primary energy (< 20 e. V) Exitation source with primary energy (100 e. V to a few ke. V) Surface phonons and vibrational modes of the adsorbates atoms and molecules Contribution of the bulk and the surface Equipment to achieve this – see S. 16 – 18 100 – 104 E, [e. V] Core level exitation corel level EELS Exitation source with primary energy of several ke. V Contribution of the bulk

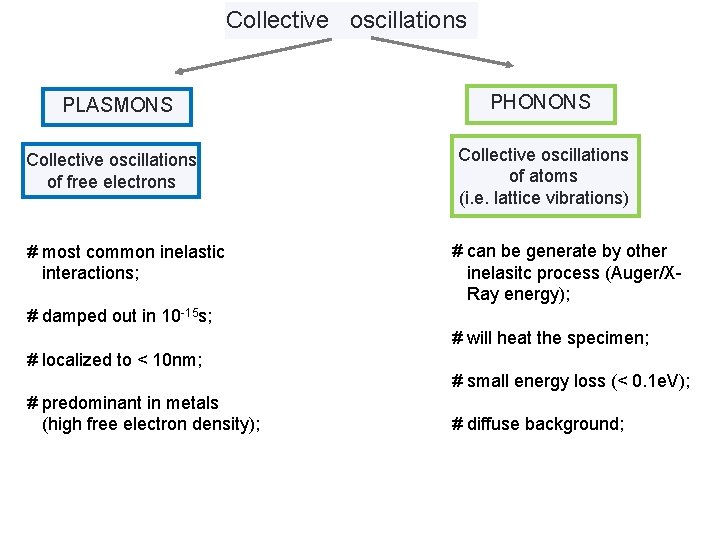
Collective oscillations PLASMONS Collective oscillations of free electrons # most common inelastic interactions; PHONONS Collective oscillations of atoms (i. e. lattice vibrations) # can be generate by other inelasitc process (Auger/XRay energy); # damped out in 10 -15 s; # will heat the specimen; # localized to < 10 nm; # small energy loss (< 0. 1 e. V); # predominant in metals (high free electron density); # diffuse background;

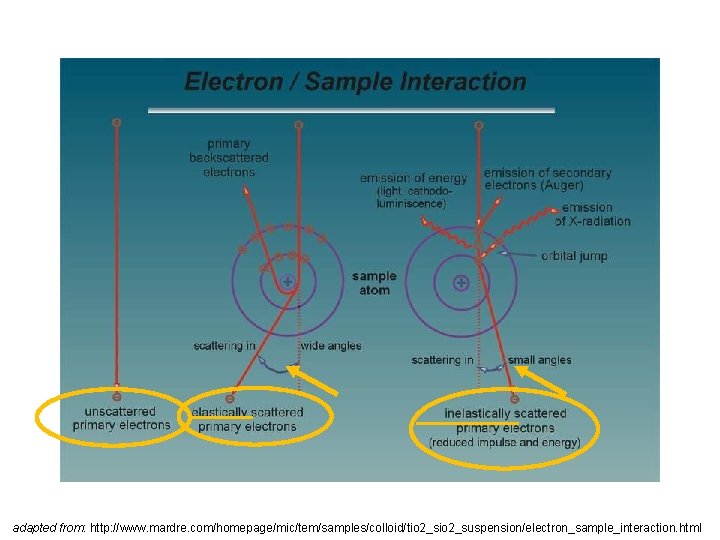
adapted from: http: //www. mardre. com/homepage/mic/tem/samples/colloid/tio 2_suspension/electron_sample_interaction. html

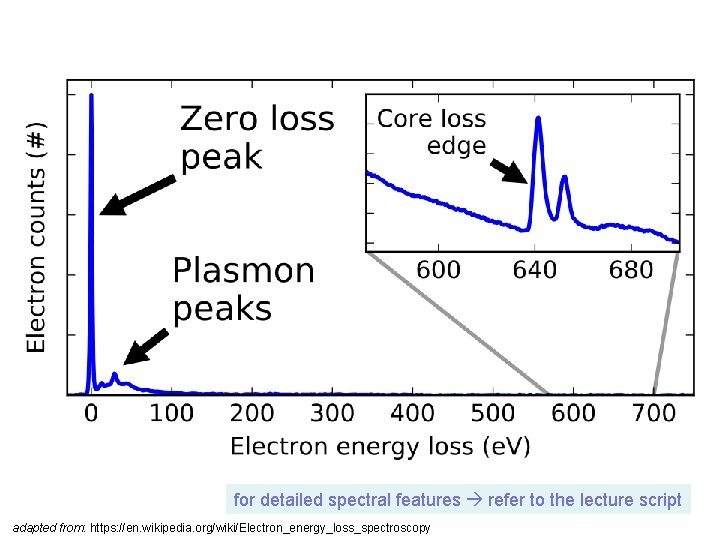
for detailed spectral features refer to the lecture script adapted from: https: //en. wikipedia. org/wiki/Electron_energy_loss_spectroscopy

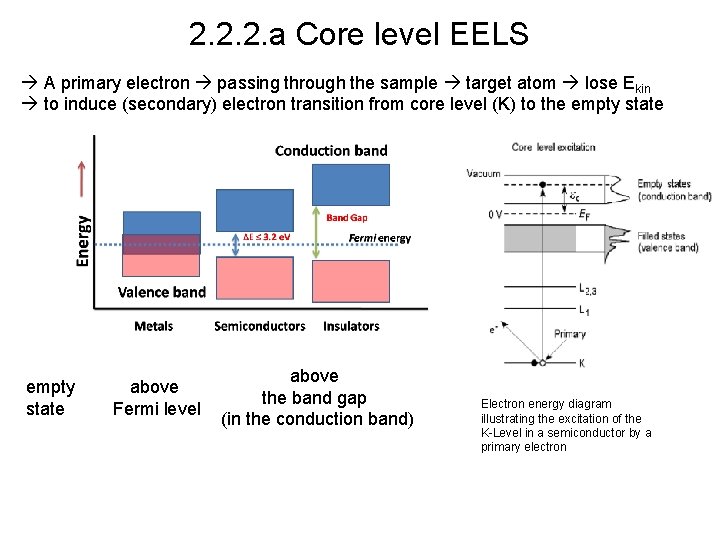
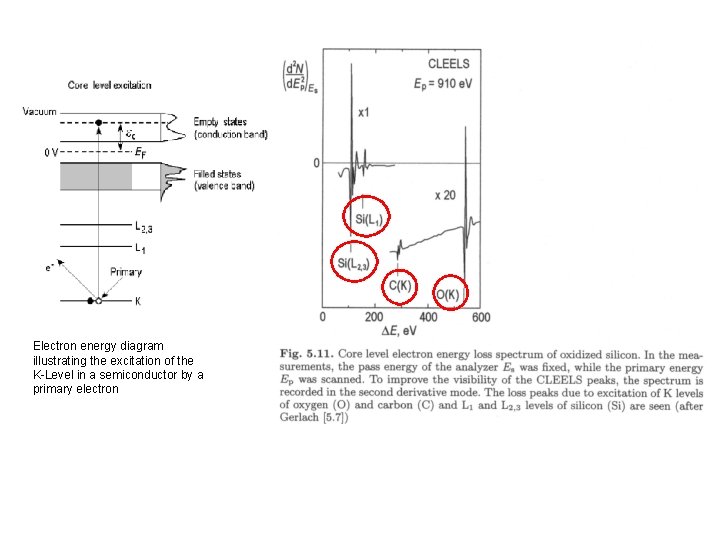
2. 2. 2. a Core level EELS A primary electron passing through the sample target atom lose Ekin to induce (secondary) electron transition from core level (K) to the empty state above Fermi level above the band gap (in the conduction band) Electron energy diagram illustrating the excitation of the K-Level in a semiconductor by a primary electron

Electron energy diagram illustrating the excitation of the K-Level in a semiconductor by a primary electron

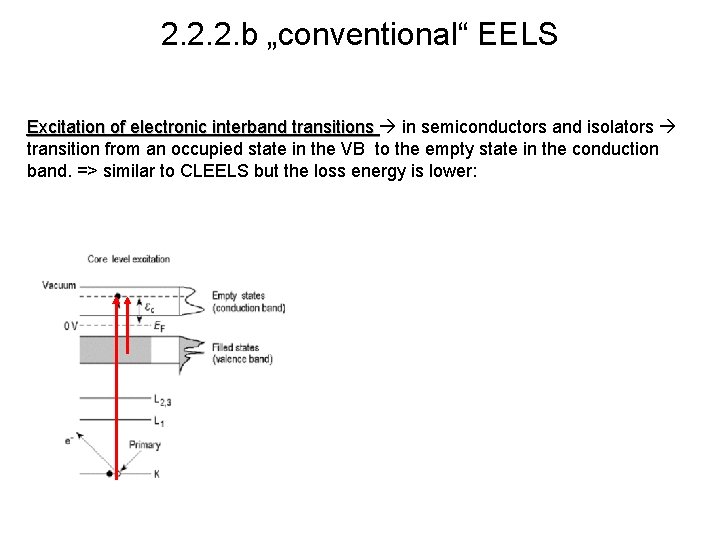
2. 2. 2. b „conventional“ EELS Excitation of electronic interband transitions in semiconductors and isolators transition from an occupied state in the VB to the empty state in the conduction band. => similar to CLEELS but the loss energy is lower:

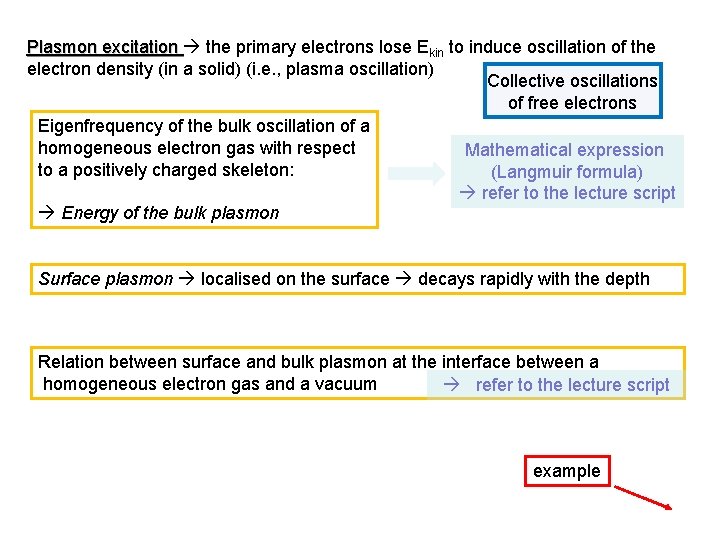
Plasmon excitation the primary electrons lose Ekin to induce oscillation of the electron density (in a solid) (i. e. , plasma oscillation) Collective oscillations of free electrons Eigenfrequency of the bulk oscillation of a homogeneous electron gas with respect Mathematical expression to a positively charged skeleton: (Langmuir formula) refer to the lecture script Energy of the bulk plasmon Surface plasmon localised on the surface decays rapidly with the depth Relation between surface and bulk plasmon at the interface between a homogeneous electron gas and a vacuum refer to the lecture script example

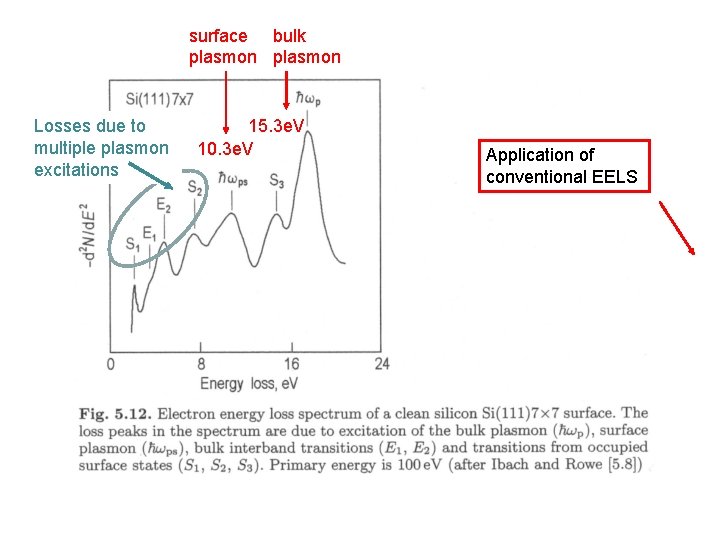
surface bulk plasmon Losses due to multiple plasmon excitations 15. 3 e. V 10. 3 e. V Application of conventional EELS

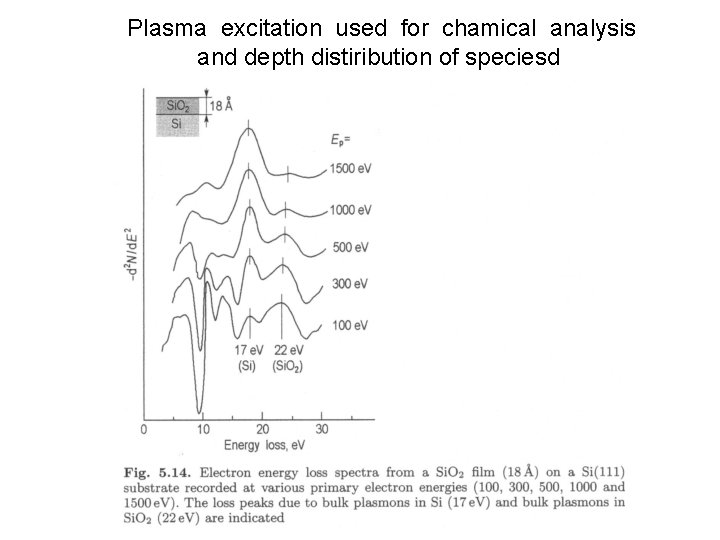
„conventional“ EELS - application # Electron denstiy determination Langmuir Formula densitiy of electrons participaiting in plasma oscillations (VB in semiconductors) ! mass of the electron constant electron density throughout the whole probing depth # Chemical analysis Electron density – characterisitc for a given solid plasmon loss energy identification of surface species Example next slide # Analysis of depth distribution of species Depends on the energy of the primary electrons Example next slide

Plasma excitation used for chamical analysis and depth distiribution of speciesd

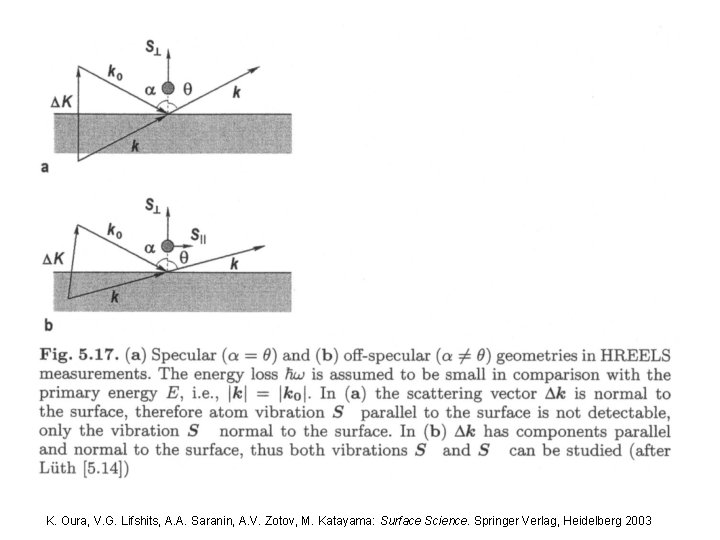
2. 2. 2. c High–resolution EELS already known: HREELS tool used in a surface science The inelastic scattering of electrons from surfaces is utilized to study electronic excitations or vibrational modes of the surface or molecules adsorbed to a surface. Hence in contrast to other EELS, HREEL deals with small energy losses in the range of 10 -3 e. V to 1 e. V. It plays an important role in the investigation of: # surface strucutre; # catalysis; # dispersion of surface phonons; # motitoring of epitaxial growtn; experimental set–up

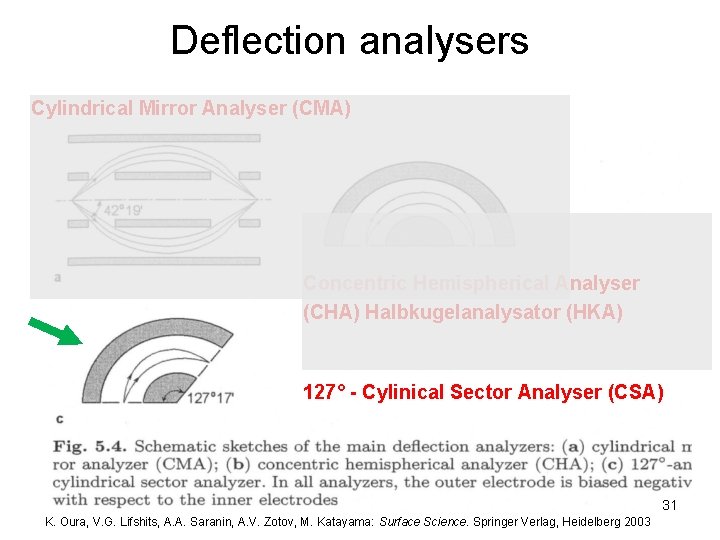
Deflection analysers Cylindrical Mirror Analyser (CMA) Concentric Hemispherical Analyser (CHA) Halbkugelanalysator (HKA) 127° - Cylinical Sector Analyser (CSA) 31 K. Oura, V. G. Lifshits, A. A. Saranin, A. V. Zotov, M. Katayama: Surface Science. Springer Verlag, Heidelberg 2003

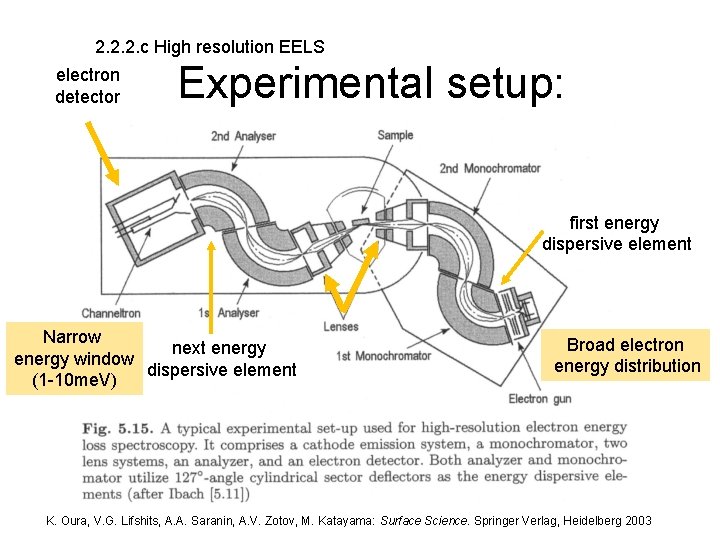
2. 2. 2. c High resolution EELS electron detector Experimental setup: first energy dispersive element Narrow next energy window dispersive element (1 -10 me. V) Broad electron energy distribution K. Oura, V. G. Lifshits, A. A. Saranin, A. V. Zotov, M. Katayama: Surface Science. Springer Verlag, Heidelberg 2003

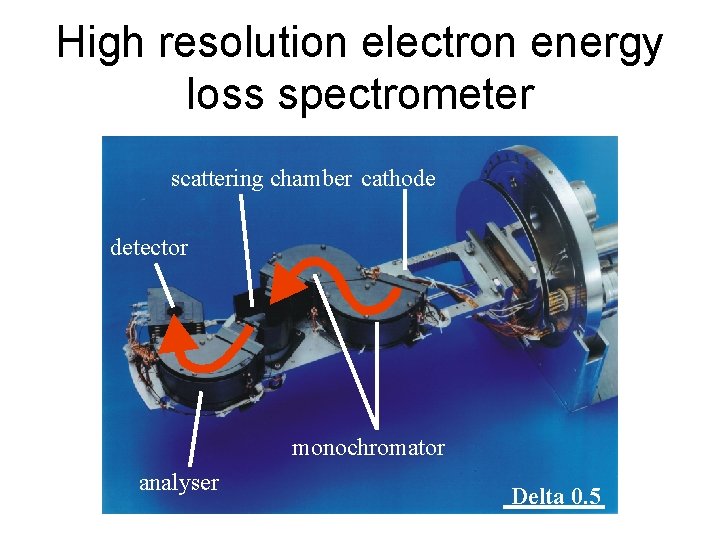
High resolution electron energy loss spectrometer scattering chamber cathode detector monochromator analyser Delta 0. 5

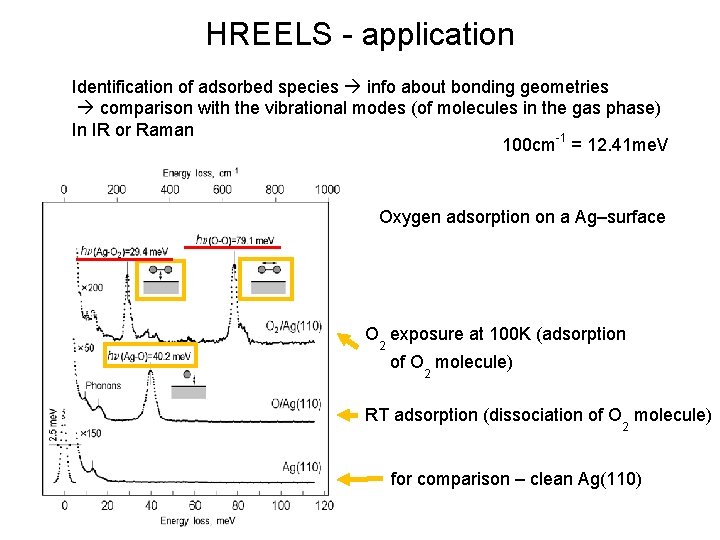
HREELS - application Identification of adsorbed species info about bonding geometries comparison with the vibrational modes (of molecules in the gas phase) In IR or Raman 100 cm-1 = 12. 41 me. V Oxygen adsorption on a Ag–surface O exposure at 100 K (adsorption 2 of O molecule) 2 RT adsorption (dissociation of O molecule) 2 for comparison – clean Ag(110)

Identification of the spatial orientation of the adsorbed molecules refer to the lecture script

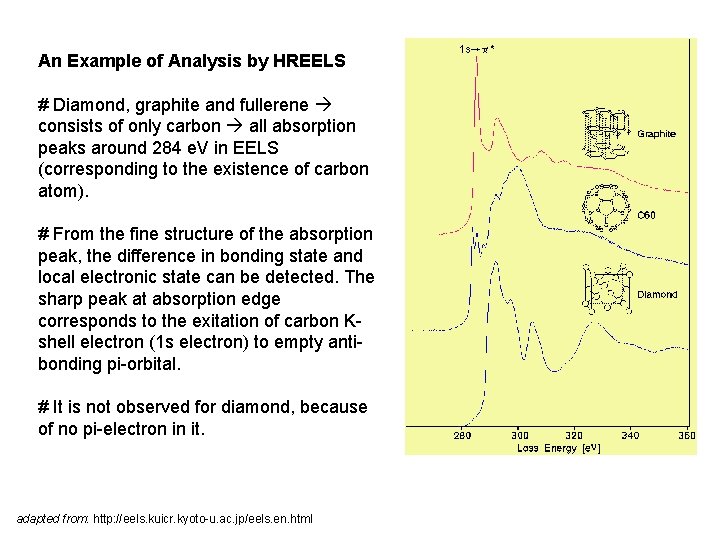
An Example of Analysis by HREELS # Diamond, graphite and fullerene consists of only carbon all absorption peaks around 284 e. V in EELS (corresponding to the existence of carbon atom). # From the fine structure of the absorption peak, the difference in bonding state and local electronic state can be detected. The sharp peak at absorption edge corresponds to the exitation of carbon Kshell electron (1 s electron) to empty antibonding pi-orbital. # It is not observed for diamond, because of no pi-electron in it. adapted from: http: //eels. kuicr. kyoto-u. ac. jp/eels. en. html

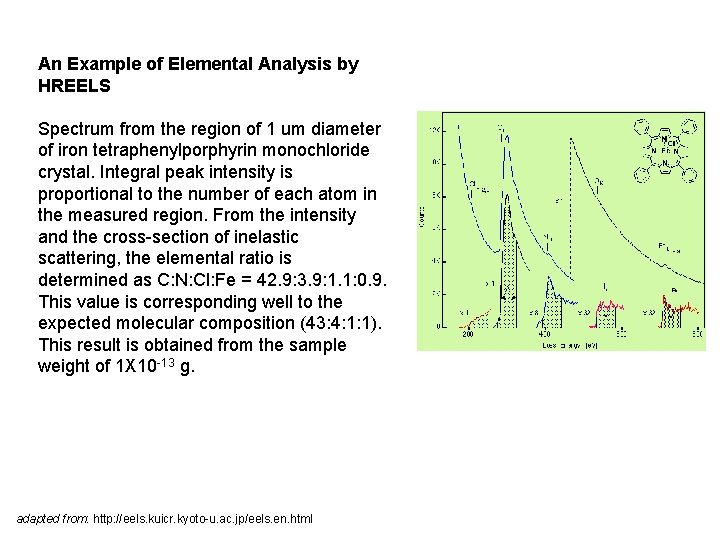
An Example of Elemental Analysis by HREELS Spectrum from the region of 1 um diameter of iron tetraphenylporphyrin monochloride crystal. Integral peak intensity is proportional to the number of each atom in the measured region. From the intensity and the cross-section of inelastic scattering, the elemental ratio is determined as C: N: Cl: Fe = 42. 9: 3. 9: 1. 1: 0. 9. This value is corresponding well to the expected molecular composition (43: 4: 1: 1). This result is obtained from the sample weight of 1 X 10 -13 g. adapted from: http: //eels. kuicr. kyoto-u. ac. jp/eels. en. html

K. Oura, V. G. Lifshits, A. A. Saranin, A. V. Zotov, M. Katayama: Surface Science. Springer Verlag, Heidelberg 2003