2 12 Delocalized vs Localized Localized electrons are




- Slides: 23

2. 12 Delocalized vs. Localized • • Localized – electrons are NOT in resonance Delocalized – electrons ARE in resonance – • Delocalization increases stability There a couple ways to recognize electrons that are delocalized through resonance? 1. To be delocalized, electrons must exist in an unhybridized p orbital that can overlap with p orbitals on neighboring atoms 2. To be delocalized, electrons must be on an sp or sp 2 hybridized atom Copyright 2012 John Wiley & Sons, Inc. 1 Klein, Organic Chemistry 2 e

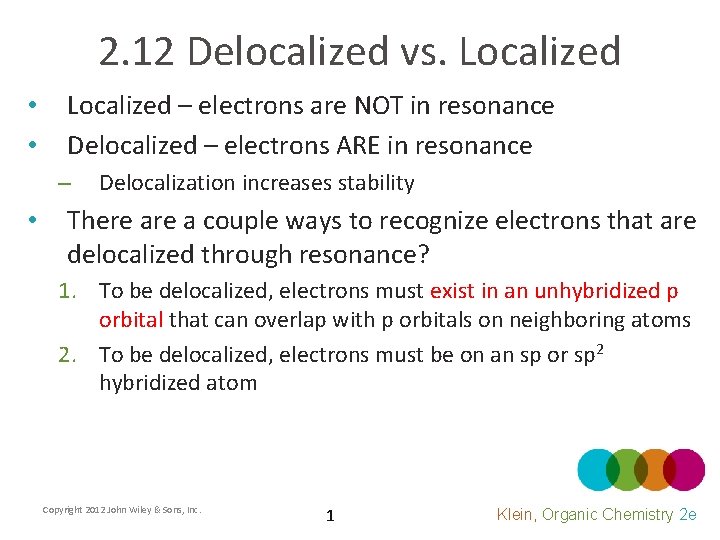
2. 12 Delocalized vs. Localized • Does the delocalization of the electrons in the amide create a more or less stable contributor? • Delocalization must be a very stabilizing force if it can favor sp 2 hybridization over sp 3 even when the delocalization doesn’t involve two equally stable contributors Copyright 2012 John Wiley & Sons, Inc. 2 Klein, Organic Chemistry 2 e

2. 12 Delocalized vs. Localized • • To allow for delocalization (resonance), some atoms that would normally be sp 3 will become sp 2 hybridized Calculate the steric number and predict the hybridization for Nitrogen atom of an amide If the Nitrogen atom were sp 3, its lone pair of electrons couldn’t engage in resonance The Nitrogen of an amide will be sp 2 hybridized – – In this case, the stability of the delocalization outweighs the stability difference between sp 2 and sp 3 Why is sp 2 generally less stable than sp 3 ? Copyright 2012 John Wiley & Sons, Inc. 3 Klein, Organic Chemistry 2 e

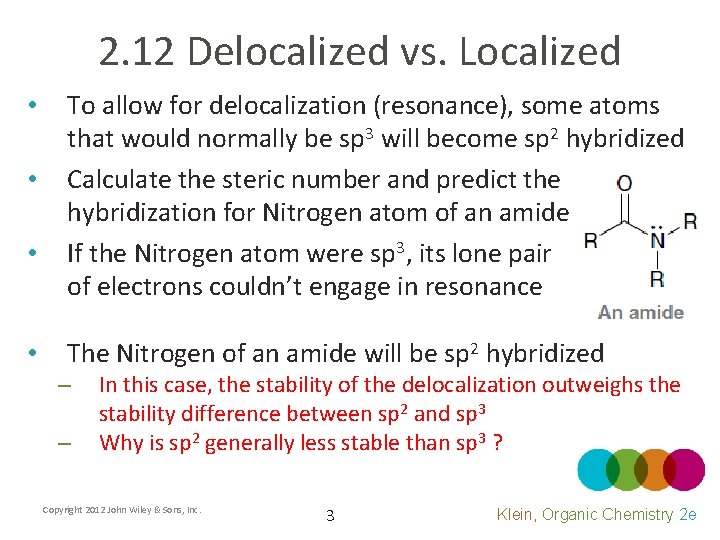
2. 12 Delocalized vs. Localized • The sp 2 hybridization of the nitrogen atom causes it to be trigonal planar rather than tetrahedral • To be delocalized, all three atoms involved MUST have p orbitals overlapping Copyright 2012 John Wiley & Sons, Inc. 4 Klein, Organic Chemistry 2 e

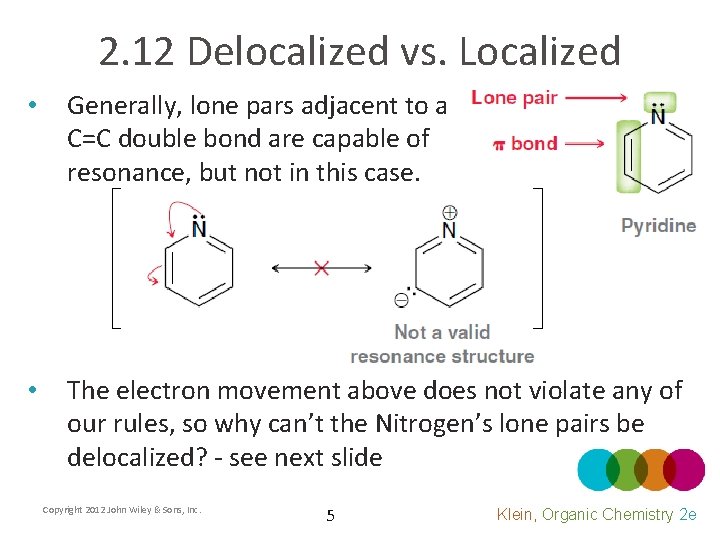
2. 12 Delocalized vs. Localized • Generally, lone pars adjacent to a C=C double bond are capable of resonance, but not in this case. • The electron movement above does not violate any of our rules, so why can’t the Nitrogen’s lone pairs be delocalized? - see next slide Copyright 2012 John Wiley & Sons, Inc. 5 Klein, Organic Chemistry 2 e

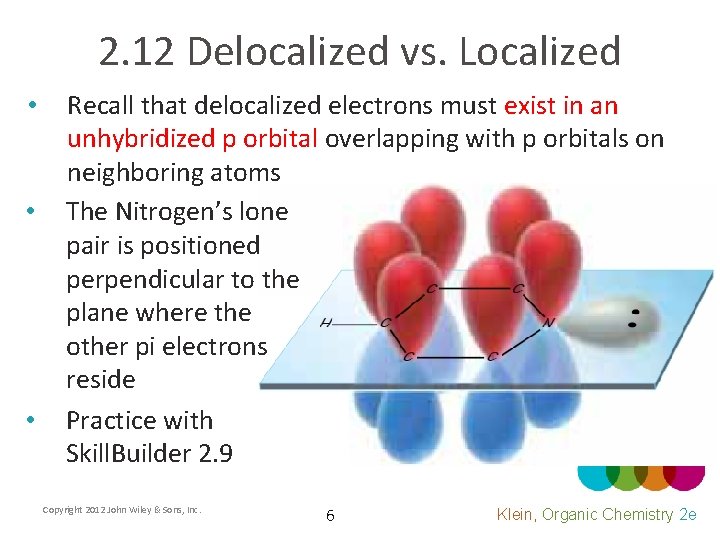
2. 12 Delocalized vs. Localized • • • Recall that delocalized electrons must exist in an unhybridized p orbital overlapping with p orbitals on neighboring atoms The Nitrogen’s lone pair is positioned perpendicular to the plane where the other pi electrons reside Practice with Skill. Builder 2. 9 Copyright 2012 John Wiley & Sons, Inc. 6 Klein, Organic Chemistry 2 e

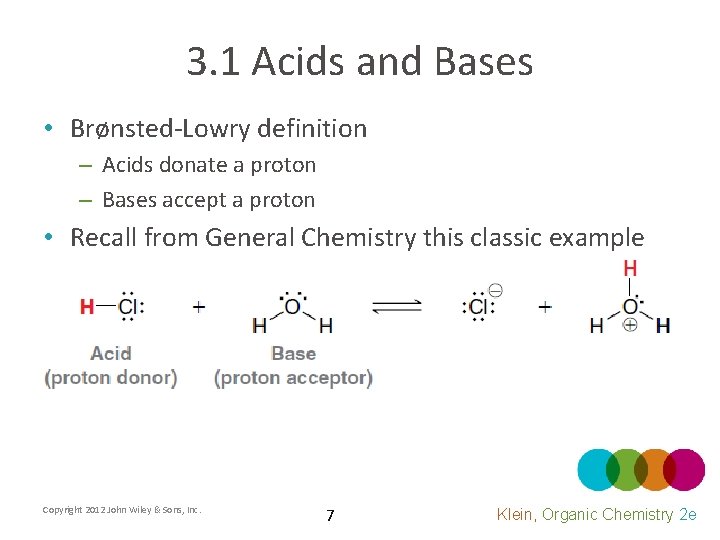
3. 1 Acids and Bases • Brønsted-Lowry definition – Acids donate a proton – Bases accept a proton • Recall from General Chemistry this classic example Copyright 2012 John Wiley & Sons, Inc. 7 Klein, Organic Chemistry 2 e

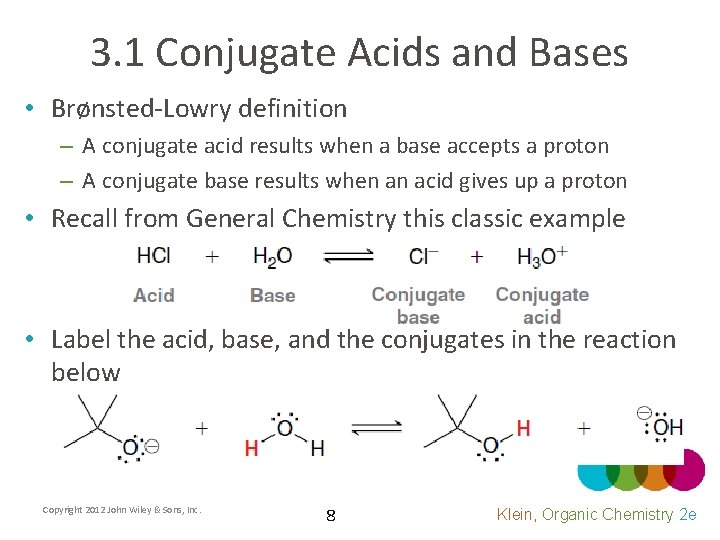
3. 1 Conjugate Acids and Bases • Brønsted-Lowry definition – A conjugate acid results when a base accepts a proton – A conjugate base results when an acid gives up a proton • Recall from General Chemistry this classic example • Label the acid, base, and the conjugates in the reaction below Copyright 2012 John Wiley & Sons, Inc. 8 Klein, Organic Chemistry 2 e

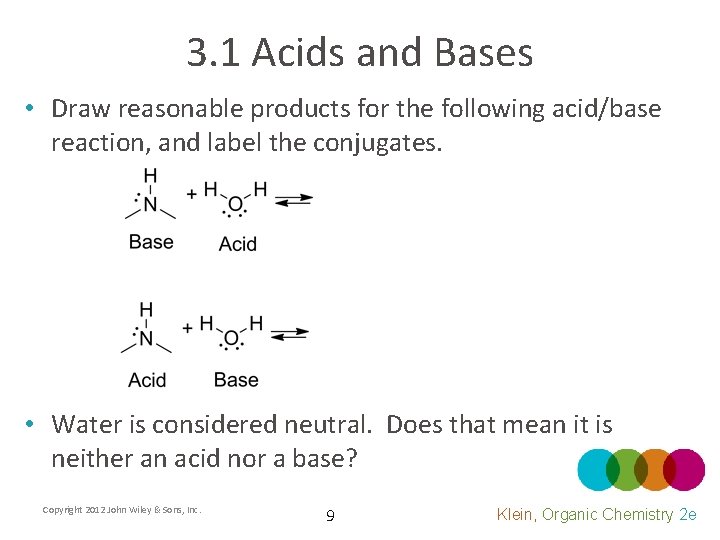
3. 1 Acids and Bases • Draw reasonable products for the following acid/base reaction, and label the conjugates. • Water is considered neutral. Does that mean it is neither an acid nor a base? Copyright 2012 John Wiley & Sons, Inc. 9 Klein, Organic Chemistry 2 e

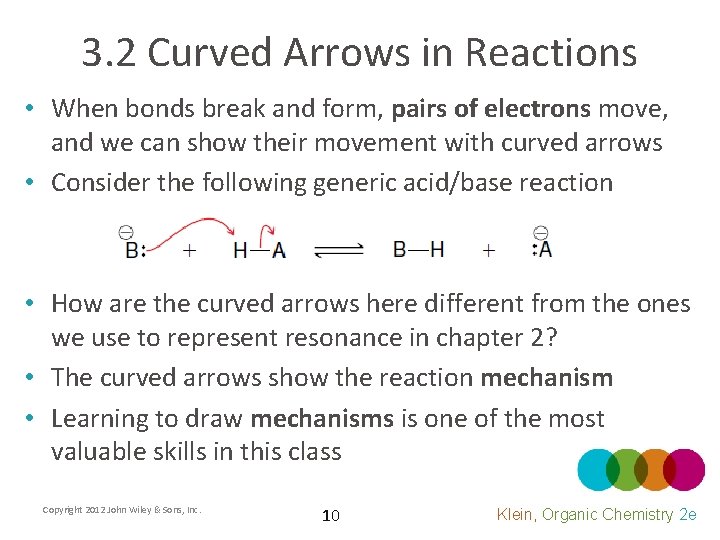
3. 2 Curved Arrows in Reactions • When bonds break and form, pairs of electrons move, and we can show their movement with curved arrows • Consider the following generic acid/base reaction • How are the curved arrows here different from the ones we use to represent resonance in chapter 2? • The curved arrows show the reaction mechanism • Learning to draw mechanisms is one of the most valuable skills in this class Copyright 2012 John Wiley & Sons, Inc. 10 Klein, Organic Chemistry 2 e

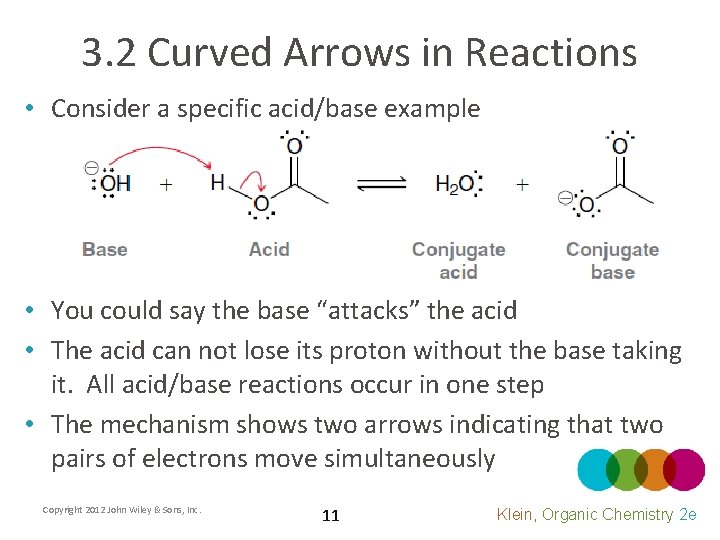
3. 2 Curved Arrows in Reactions • Consider a specific acid/base example • You could say the base “attacks” the acid • The acid can not lose its proton without the base taking it. All acid/base reactions occur in one step • The mechanism shows two arrows indicating that two pairs of electrons move simultaneously Copyright 2012 John Wiley & Sons, Inc. 11 Klein, Organic Chemistry 2 e

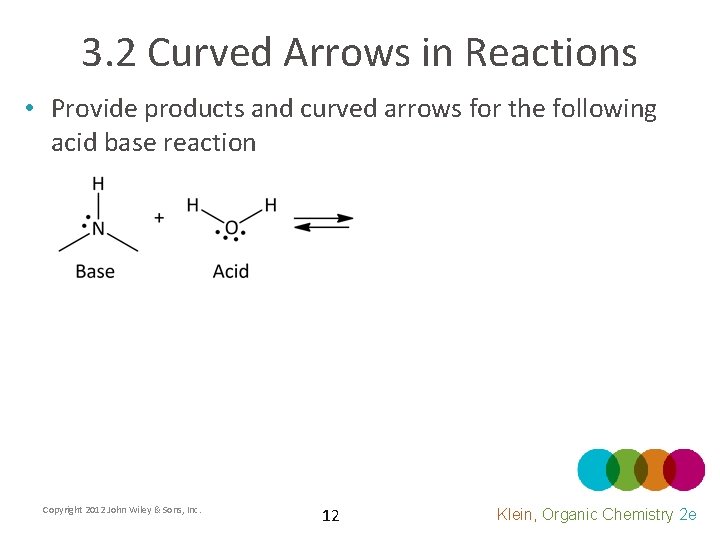
3. 2 Curved Arrows in Reactions • Provide products and curved arrows for the following acid base reaction Copyright 2012 John Wiley & Sons, Inc. 12 Klein, Organic Chemistry 2 e

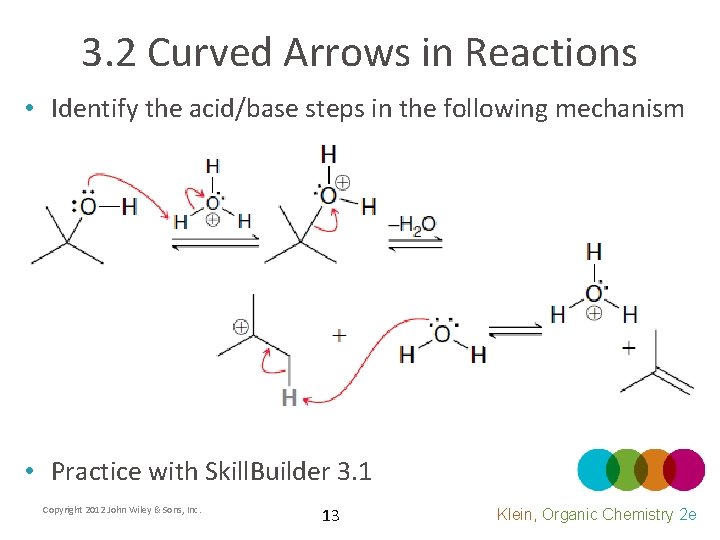
3. 2 Curved Arrows in Reactions • Identify the acid/base steps in the following mechanism • Practice with Skill. Builder 3. 1 Copyright 2012 John Wiley & Sons, Inc. 13 Klein, Organic Chemistry 2 e

3. 3 Quantifying Acidity • Recall from General Chemistry, how do “strong” acids/bases differ from “weak” acids/bases? • The strength of an acid or base is important to predict how reactions will progress – We will learn to do Quantitative strength analysis – using p. Ka values to compare the strengths of acids – We will learn to do Qualitative strength analysis – comparing the general stability of structures. Copyright 2012 John Wiley & Sons, Inc. 14 Klein, Organic Chemistry 2 e

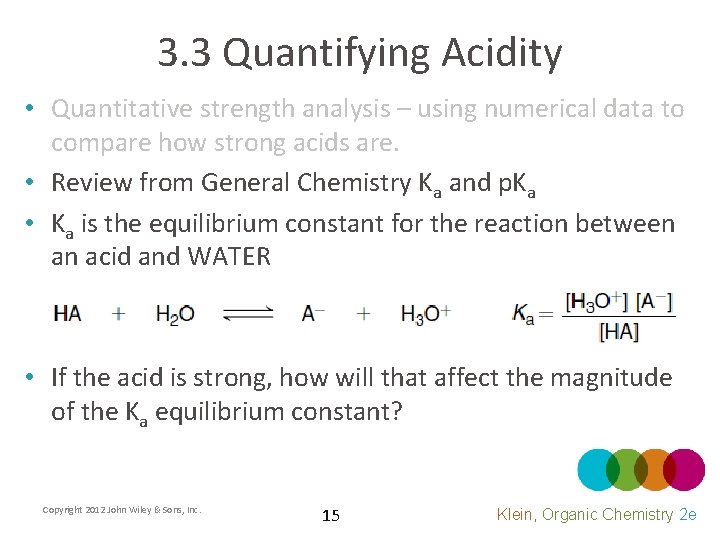
3. 3 Quantifying Acidity • Quantitative strength analysis – using numerical data to compare how strong acids are. • Review from General Chemistry Ka and p. Ka • Ka is the equilibrium constant for the reaction between an acid and WATER • If the acid is strong, how will that affect the magnitude of the Ka equilibrium constant? Copyright 2012 John Wiley & Sons, Inc. 15 Klein, Organic Chemistry 2 e

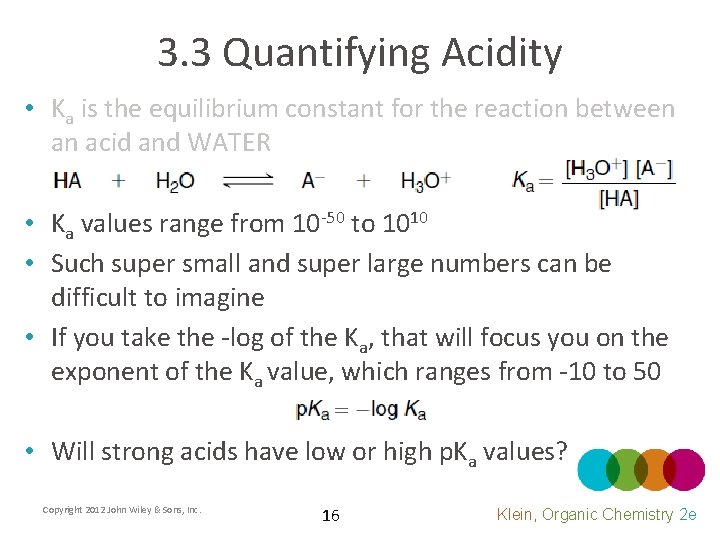
3. 3 Quantifying Acidity • Ka is the equilibrium constant for the reaction between an acid and WATER • Ka values range from 10 -50 to 1010 • Such super small and super large numbers can be difficult to imagine • If you take the -log of the Ka, that will focus you on the exponent of the Ka value, which ranges from -10 to 50 • Will strong acids have low or high p. Ka values? Copyright 2012 John Wiley & Sons, Inc. 16 Klein, Organic Chemistry 2 e

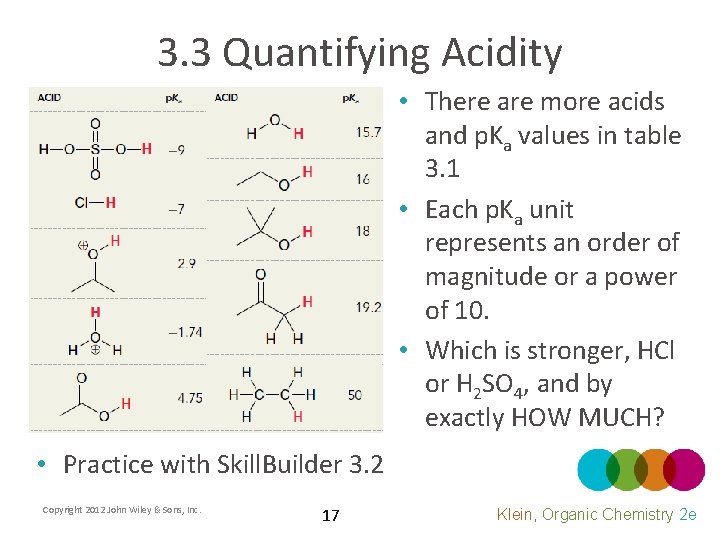
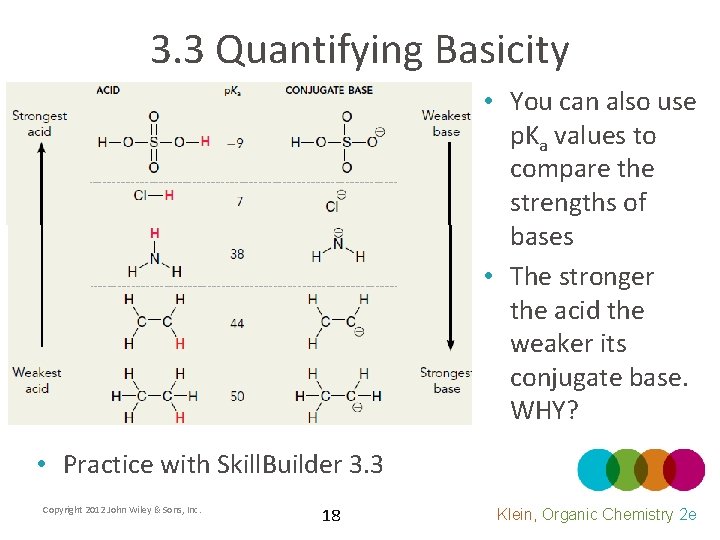
3. 3 Quantifying Acidity • There are more acids and p. Ka values in table 3. 1 • Each p. Ka unit represents an order of magnitude or a power of 10. • Which is stronger, HCl or H 2 SO 4, and by exactly HOW MUCH? • Practice with Skill. Builder 3. 2 Copyright 2012 John Wiley & Sons, Inc. 17 Klein, Organic Chemistry 2 e

3. 3 Quantifying Basicity • You can also use p. Ka values to compare the strengths of bases • The stronger the acid the weaker its conjugate base. WHY? • Practice with Skill. Builder 3. 3 Copyright 2012 John Wiley & Sons, Inc. 18 Klein, Organic Chemistry 2 e

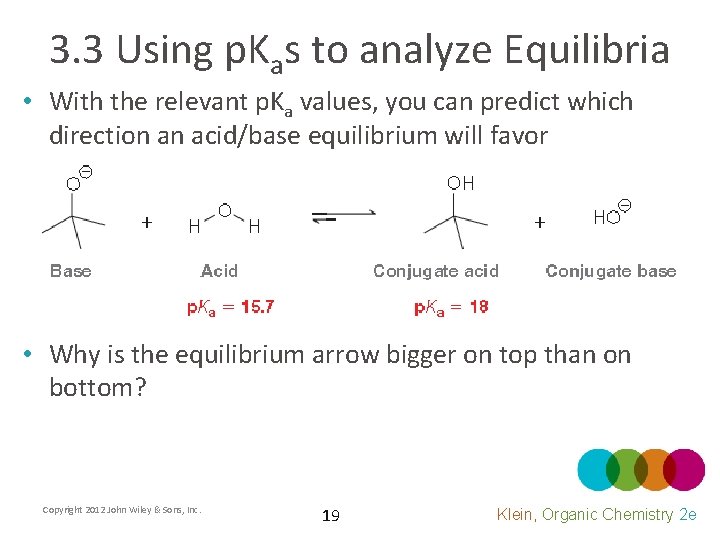
3. 3 Using p. Kas to analyze Equilibria • With the relevant p. Ka values, you can predict which direction an acid/base equilibrium will favor • Why is the equilibrium arrow bigger on top than on bottom? Copyright 2012 John Wiley & Sons, Inc. 19 Klein, Organic Chemistry 2 e

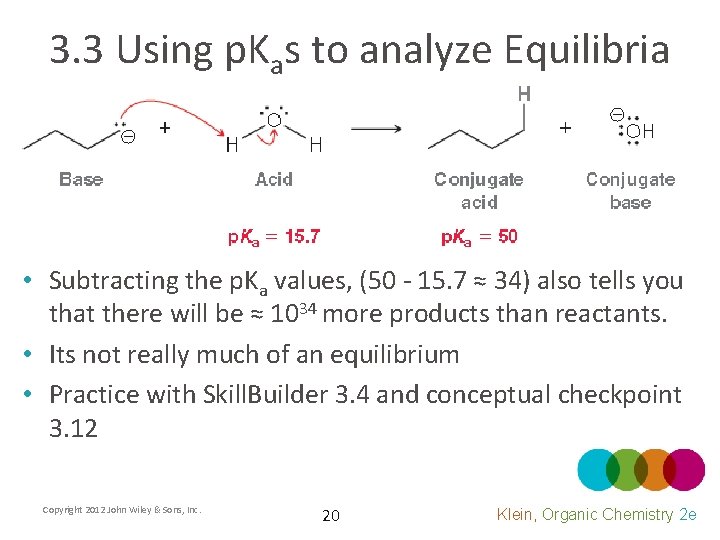
3. 3 Using p. Kas to analyze Equilibria • Subtracting the p. Ka values, (50 - 15. 7 ≈ 34) also tells you that there will be ≈ 1034 more products than reactants. • Its not really much of an equilibrium • Practice with Skill. Builder 3. 4 and conceptual checkpoint 3. 12 Copyright 2012 John Wiley & Sons, Inc. 20 Klein, Organic Chemistry 2 e

Study Guide for sections 2. 11 -2. 12, 3. 1 -3. 3 DAY 5, Terms to know: Sections 2. 11 -2. 12, 3. 1 -3. 3 resonance contributor, resonance hybrid, delocalization, conjugation, allyl, vinyl, Bronsted-Lowry acid, Bronsted-Lowry base, conjugate acid, conjugate base, mechanism, strong acid or base, weak acid or base, Ka, p. Ka, Kb, p. Kb DAY 5, Specific outcomes and skills that may be tested on exam 1: Sections 2. 11 -2. 12, 3. 1 -3. 3 • Be able to assess the relative stability of resonance contributors and draw a hybrid that accounts for stability differences in the contributors. • Be able to correctly predict the hybridization and steric number for atoms that are affected by resonance. • Given acid and base reactants, be able to predict the products for an acid/base reaction. • Be able to use curved arrows to show electron movement in acid/base reactions. • Given a reaction where products are shown, be able to determine which reactant was the acid and which was the base. • Given a mechanism with curved arrows, be able to identify acid base steps in the mechanism. • Be able to give the equilibrium expression for an acid or base reaction and show Ka, p. Ka, Kb, and p. Kb are calculated. • Be able to explain how the value for Ka, p. Ka, Kb, and p. Kb related to the strength of a corresponding acid or base. • Given the p. Ka for an acid, be able to predict whether reactions involving that acid will favor reactants or products at equilibrium and by how much. 21 Klein, Organic. Chemistry 2 e 2 e Klein, Organic

Practice Problems for sections 2. 11 -2. 12, 3. 1 -3. 3 Complete these problems outside of class until you are confident you have learned the SKILLS in this section outlined on the study guide and we will review some of them next class period. 2. 33 2. 34 2. 35 2. 36 2. 47 2. 48 2. 51 2. 53 2. 55 2. 56 2. 57 2. 61 2. 62 2. 63 2. 65 2. 66 2. 67 3. 1 3. 2 3. 3 3. 4 3. 5 3. 6 3. 7 3. 9 3. 10 3. 11 3. 12 22 Klein, Organic. Chemistry 2 e 2 e Klein, Organic

Prep for day 6 Must Watch videos: https: //www. youtube. com/watch? v=v. AYo. AH 98 Gag (relationship between solvation and strength) https: //www. youtube. com/watch? v=m. Zq 8 ab. Ozu. Qw (relationship between charge and strength) https: //www. youtube. com/watch? v=vw 8 urca. BOBk (relationship between atom and strength) https: //www. youtube. com/watch? v=Sg. Ljwza. Ah. TA (relationship between resonance and strength) https: //www. youtube. com/watch? v=Ko. C-Pin. XWCA (relationship between induction and strength) https: //www. youtube. com/watch? v=TNZOYaol. Pr. I (relationship between orbital and strength) https: //www. youtube. com/watch? v=ss 7 Ap-6 b. FYw (Lewis acid/base) Other helpful videos: https: //www. youtube. com/playlist? list=PLu 5 d. IVYg. NSyv 8 E 2 YXt. Kub. FJZRj_f. C 7 m. R- (acid base concepts playlist) http: //ps. uci. edu/content/chem-51 a-organic-chemistry (lecture 6) Read sections 3. 4 -3. 9 23 Klein, Organic. Chemistry 2 e 2 e Klein, Organic