2 1 The Nature of Matter LT Today







- Slides: 28

2. 1 The Nature of Matter • LT: Today I will… – Identify the three subatomic particles found in atoms • Entry Task: gr – Describe the two ab a text b ook and main types of a copy o chemical bonds f the people search from th e front of the roo m. Word Atom Nucleus Electron Proton Element Compound Ionic bond Covalent bond Valence electrons

FQ: What are atoms and what are they made of? ET: take out your vocabulary flipper from Friday. Review all the terms.

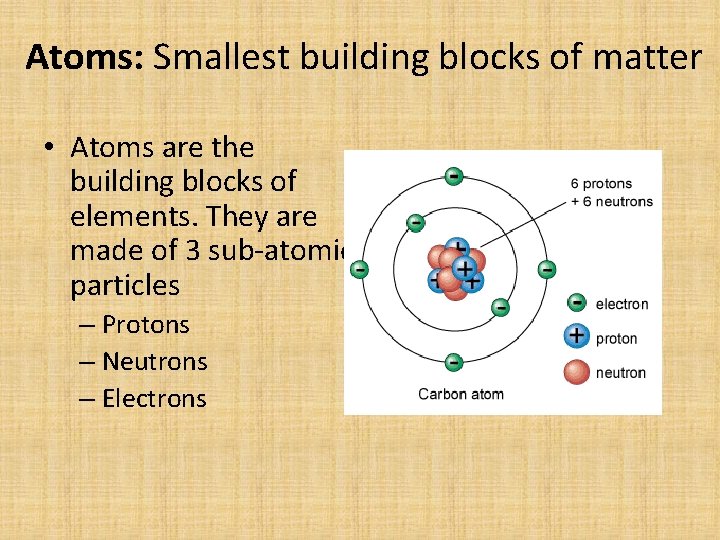
Atoms: Smallest building blocks of matter • Atoms are the building blocks of elements. They are made of 3 sub-atomic particles – Protons – Neutrons – Electrons

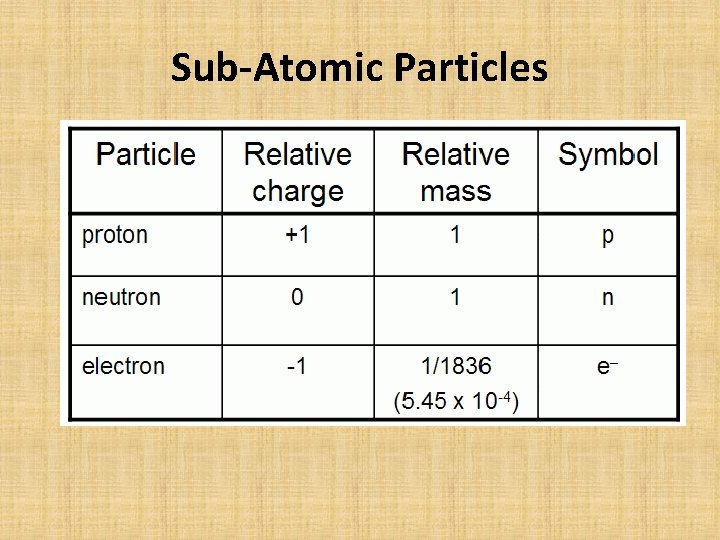
Sub-Atomic Particles


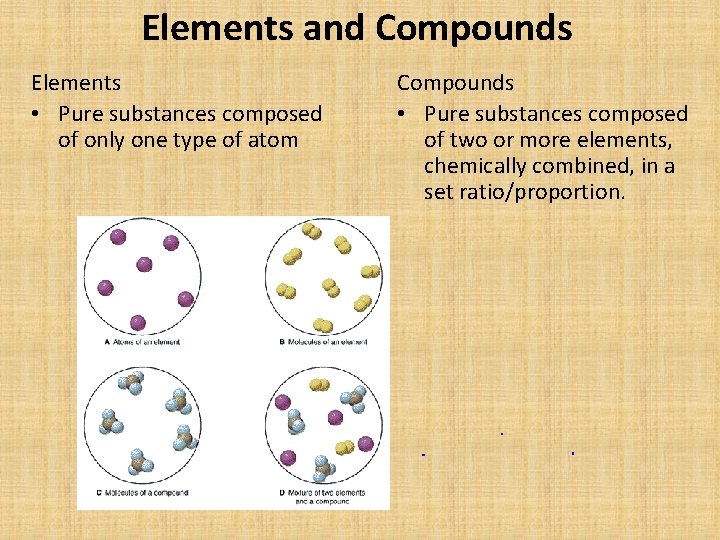
Elements and Compounds Elements • Pure substances composed of only one type of atom Compounds • Pure substances composed of two or more elements, chemically combined, in a set ratio/proportion.

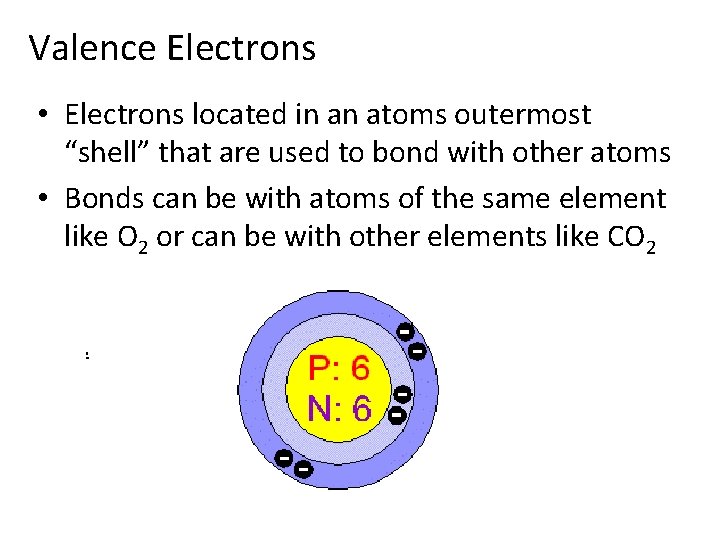
Valence Electrons • Electrons located in an atoms outermost “shell” that are used to bond with other atoms • Bonds can be with atoms of the same element like O 2 or can be with other elements like CO 2

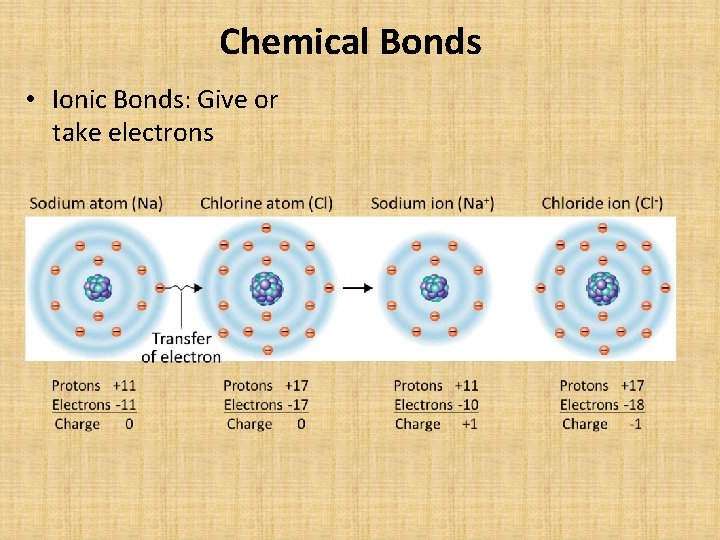
Chemical Bonds • Ionic Bonds: Give or take electrons

Chemical Bonds • Covalent bonds share electrons

EXIT TICKET • Draw a basic atom and include the following – The 3 different sub-atomic particles – The charges next to those particles Note: the number of each does not matter

Homework • Read section 2. 3 • Take notes on the following – What is a… • • Protein Carbohydrate Lipid Nucleic acid

Friday 11/11 - 2. 2 Properties of Water • LT: Today I will… – Explain polarity – Explain what makes a water molecule polar • ET: What is responsible for the making and breaking of chemical bonds? Please explain your answer Your POGIL is your notes for this section and should be in your notebook

2. 3 – Carbon (organic) Compounds • Lt: Today I will… – Describe the unique qualities of carbon – Describe the structures and functions of each of the four groups of macromolecules • ET: What elements does carbon bond with that make up life’s molecules?

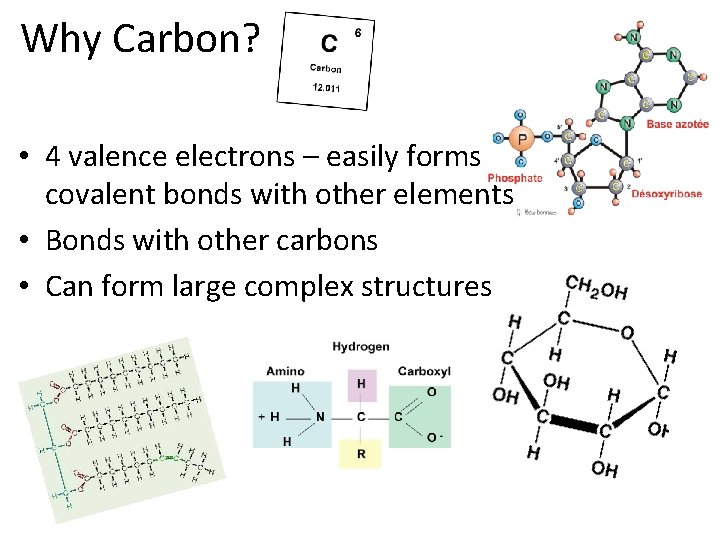
Why Carbon? • 4 valence electrons – easily forms covalent bonds with other elements • Bonds with other carbons • Can form large complex structures

Macromolecules (large molecules) • Monomers – , smaller units that when linked together, form s le u c le o m r e g r la (macro). • Polymers – a macromolecu le formed from linked m onomers – The monome rs may be identical or th ey may be different


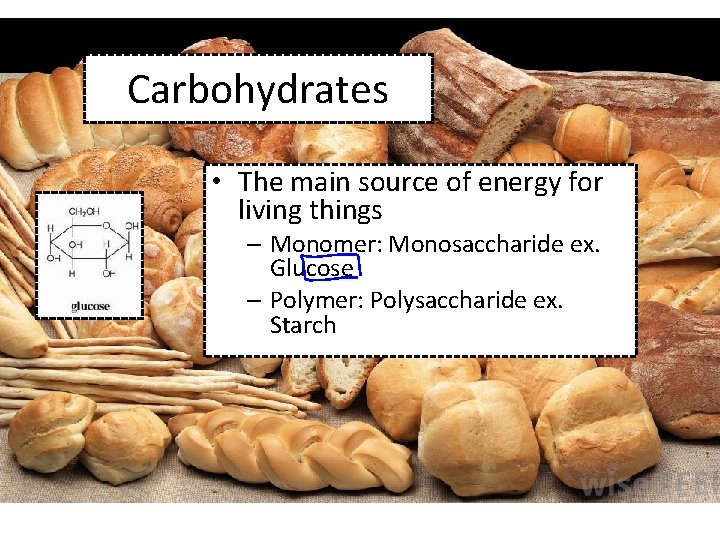
Carbohydrates • The main source of energy for living things – Monomer: Monosaccharide ex. Glucose – Polymer: Polysaccharide ex. Starch

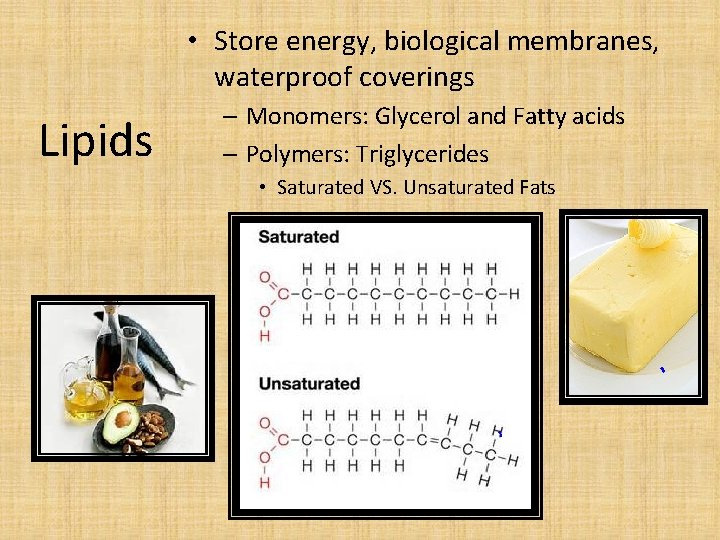
• Store energy, biological membranes, waterproof coverings Lipids – Monomers: Glycerol and Fatty acids – Polymers: Triglycerides • Saturated VS. Unsaturated Fats

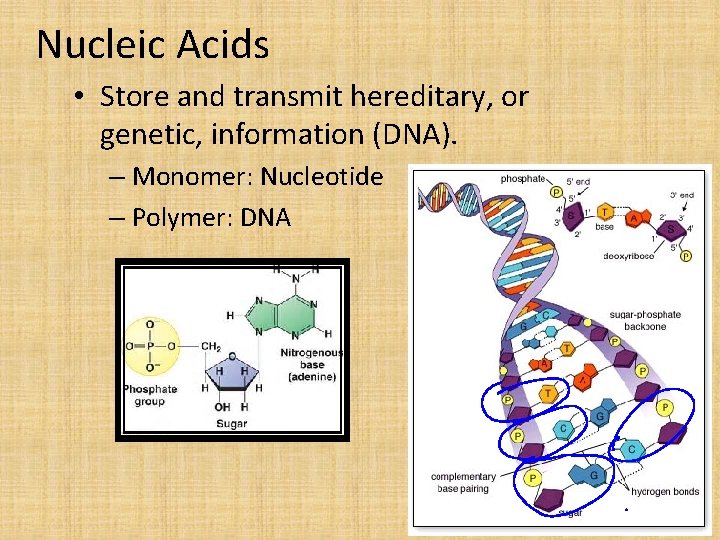
Nucleic Acids • Store and transmit hereditary, or genetic, information (DNA). – Monomer: Nucleotide – Polymer: DNA

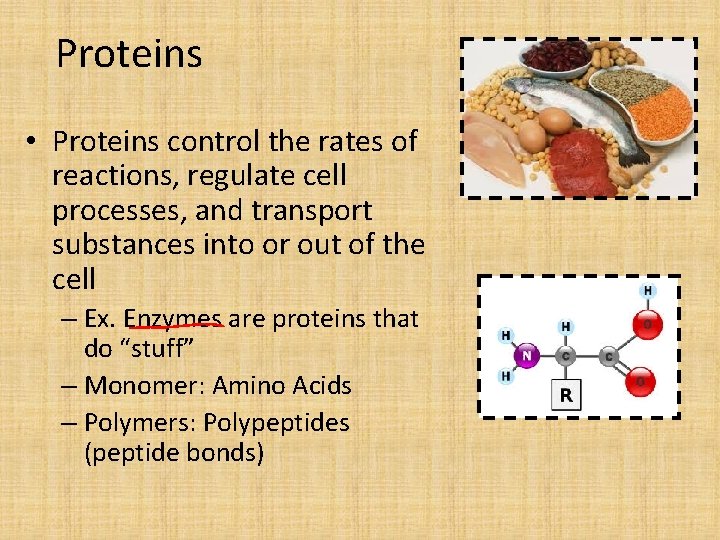
Proteins • Proteins control the rates of reactions, regulate cell processes, and transport substances into or out of the cell – Ex. Enzymes are proteins that do “stuff” – Monomer: Amino Acids – Polymers: Polypeptides (peptide bonds)

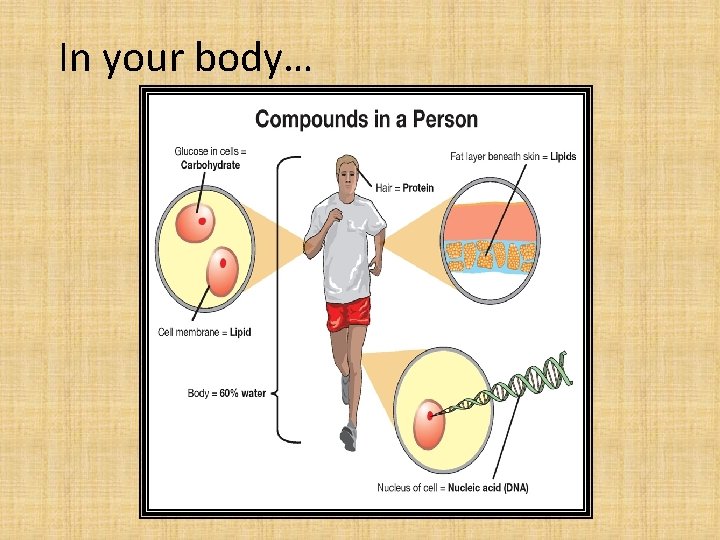
In your body…

Enzymes and Chemical reactions • LT: Today I will – Explain why enzymes are important to living things – Identify the parts of a chemical reaction – Explain what chemical reactions do • ET: Get your conclusions out and be prepared to share them. • Write down the following words and leave room to add definitions in your notes – – – Activation energy Reactant Product Enzyme Substrate

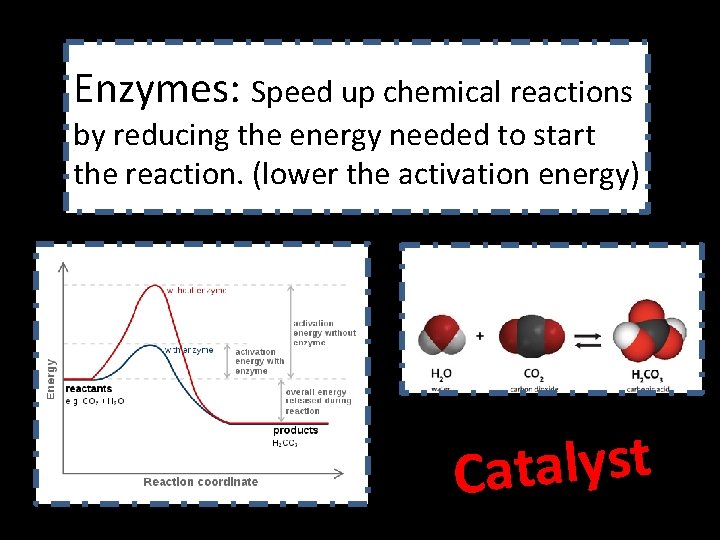
Enzymes: Speed up chemical reactions by reducing the energy needed to start the reaction. (lower the activation energy) t s y l a t Ca

Enzyme/Temperature Lab • Will work with a bin at table groups *except hot plate groups • Clean up: rinse out graduated cylinders in the sink • Safety: goggles

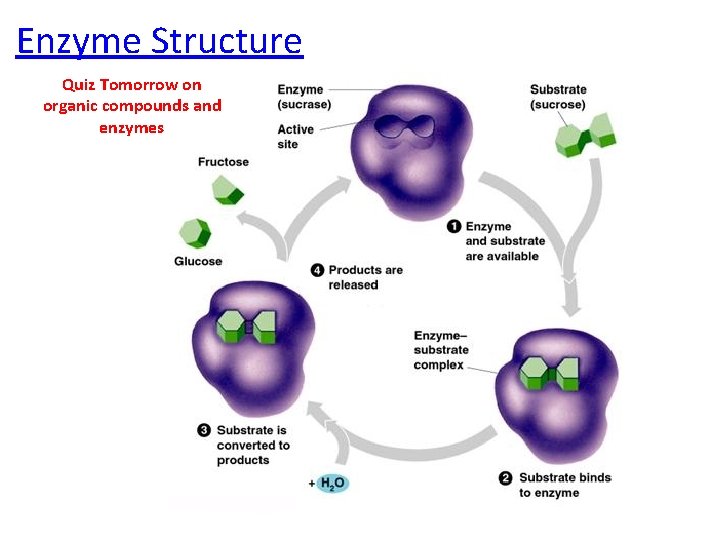
Enzyme Structure Quiz Tomorrow on organic compounds and enzymes

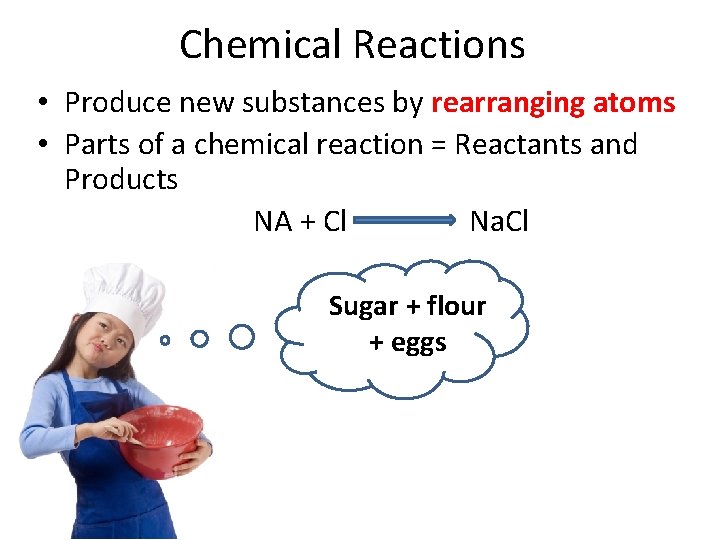
Chemical Reactions • Produce new substances by rearranging atoms • Parts of a chemical reaction = Reactants and Products NA + Cl Na. Cl Sugar + flour + eggs

Homeostasis • Maintaining a constant internal environment • How does your body do this? • It uses a process called FEEDBACK – 2 types of feedback are 1. Negative – works to maintain a baseline level ex. Your every day stuff like maintaining temperature 2. Positive – works to push way passed a baseline level ex. Rarer events like child birth

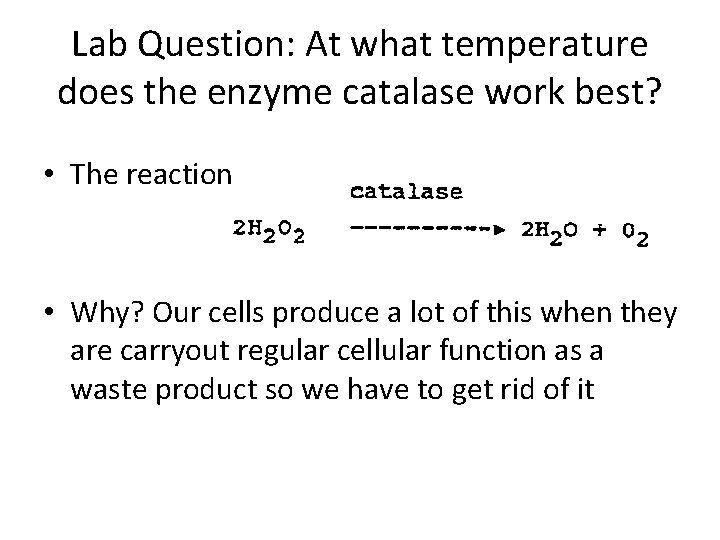
Lab Question: At what temperature does the enzyme catalase work best? • The reaction • Why? Our cells produce a lot of this when they are carryout regular cellular function as a waste product so we have to get rid of it